Inhibition of Caffeine Metabolism by Apiaceous and Rutaceae Families of Plant Products in Humans: In Vivo and In Vitro Studies
- PMID: 33995046
- PMCID: PMC8116649
- DOI: 10.3389/fphar.2021.641090
Inhibition of Caffeine Metabolism by Apiaceous and Rutaceae Families of Plant Products in Humans: In Vivo and In Vitro Studies
Abstract
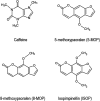
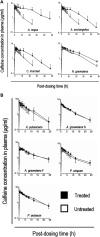
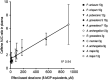
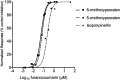
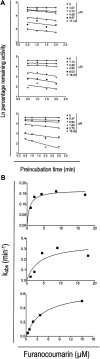
Daily consumption of caffeinated beverages is considered safe but serious health consequences do happen in some individuals. The Apiaceous and Rutaceae families of plant (ARFP) products are popular foods and medicines in the world. We previously reported significant amounts of furanocoumarin bioactive such as 8-methoxypsoralen, 5-methoxypsoralen, and isopimpinellin in ARFP products. As both caffeine and furanocoumarin bioactive are metabolized by the same hepatic CYP1A1/2 isozyme in humans, caffeine/ARFP product interactions may occur after co-administration. The objectives of the present study were to study in vivo loss of caffeine metabolizing activity by comparing the pharmacokinetics of caffeine in volunteers before and after pre-treatment with an ARFP extract, study the correlation between the decrease in hepatic CYP1A2 activity and the content of furanocoumarin bioactive in ARFP extracts, characterize CYP1A2 inactivation using in vitro incubations containing 14C-caffeine, a furanocoumarin bioactive, and human liver microsomes (HLMs), and provide a mechanistic explanation for both in vivo and in vitro data using the irreversible inhibition mechanism. The study results showed pre-treatment of volunteers with four ARFP extracts increased the area-under-the-concentration-time-curve (AUC0-inf) ratio of caffeine in the plasma ranging from 1.3 to 4.3-fold compared to the untreated volunteers indicating significant caffeine metabolism inhibition. The increases in AUC0-inf ratio also were linearly related to the effect-based doses of the furanocoumarins in the ARFP extracts, a finding which indicated caffeine metabolism inhibition was related to the content of furanocoumarin bioactive in an ARFP product. In vitro incubation studies also showed individual furanocoumarin bioactive were potent inhibitors of caffeine-N-demethylation; the IC50 for 8-methoxypsoralen 5-methoxypsoralen, and isopimpinellin were 0.09, 0.13, and 0.29 µM, respectively. In addition, CYP1A2 inactivation by individual furanocoumarin bioactive was concentration- and time-dependent involving the irreversible inhibition mechanism. The proposed irreversible inhibition mechanism was investigated further using 14C-labeled 8-methoxypsoralen and HLMs. The formation of 14C-adducts due to 14C-8-MOP-derived radioactivity bound to HLMs confirmed the irreversible inhibition of CYP1A2 activity. Thus, furanocoumarin bioactive metabolism in humans would result in reactive metabolite(s) formation inactivating CYP1A2 isozyme and inhibiting caffeine metabolism. Once the CYP1A2 isozyme was deactivated, the enzymic activity could only be regained by isozyme re-synthesis which took a long time. As a result, a single oral dose of ARFP extract administered to the human volunteers 3.0 h before still was able to inhibit caffeine metabolism.
Keywords: P450 cytochrome enzymes; caffeine; chemical mixtures; enzyme inactivation mechanism; furanocoumarin.
Copyright © 2021 Alehaideb, Sheriffdeen and Law.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Prediction of herb-drug interactions involving consumption of furanocoumarin-mixtures and cytochrome P450 1A2-mediated caffeine metabolism inhibition in humans.Saudi Pharm J. 2023 Mar;31(3):444-452. doi: 10.1016/j.jsps.2023.01.011. Epub 2023 Feb 1. Saudi Pharm J. 2023. PMID: 37026048 Free PMC article.
-
Determination of benchmark doses for linear furanocoumarin consumption associated with inhibition of cytochrome P450 1A2 isoenzyme activity in healthy human adults.Toxicol Rep. 2021 Jul 22;8:1437-1444. doi: 10.1016/j.toxrep.2021.07.013. eCollection 2021. Toxicol Rep. 2021. PMID: 34377680 Free PMC article.
-
Caffeine/Angelica dahurica and caffeine/Salvia miltiorrhiza metabolic inhibition in humans: In vitro and in vivo studies.Complement Ther Med. 2019 Oct;46:87-94. doi: 10.1016/j.ctim.2019.07.024. Epub 2019 Jul 31. Complement Ther Med. 2019. PMID: 31519293
-
Interaction of pefloxacin and enoxacin with the human cytochrome P450 enzyme CYP1A2.Clin Pharmacol Ther. 1999 Mar;65(3):262-74. doi: 10.1016/S0009-9236(99)70105-0. Clin Pharmacol Ther. 1999. PMID: 10096258 Clinical Trial.
-
Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat.Pharmacol Rep. 2008 Nov-Dec;60(6):789-97. Pharmacol Rep. 2008. PMID: 19211970 Review.
Cited by
-
Melatonin Activation by Cytochrome P450 Isozymes: How Does CYP1A2 Compare to CYP1A1?Int J Mol Sci. 2023 Feb 11;24(4):3651. doi: 10.3390/ijms24043651. Int J Mol Sci. 2023. PMID: 36835057 Free PMC article.
-
Caffeine in liver diseases: Pharmacology and toxicology.Front Pharmacol. 2022 Oct 17;13:1030173. doi: 10.3389/fphar.2022.1030173. eCollection 2022. Front Pharmacol. 2022. PMID: 36324678 Free PMC article. Review.
-
Prediction of herb-drug interactions involving consumption of furanocoumarin-mixtures and cytochrome P450 1A2-mediated caffeine metabolism inhibition in humans.Saudi Pharm J. 2023 Mar;31(3):444-452. doi: 10.1016/j.jsps.2023.01.011. Epub 2023 Feb 1. Saudi Pharm J. 2023. PMID: 37026048 Free PMC article.
-
Methoxyfuranocoumarins of Natural Origin-Updating Biological Activity Research and Searching for New Directions-A Review.Curr Issues Mol Biol. 2024 Jan 19;46(1):856-883. doi: 10.3390/cimb46010055. Curr Issues Mol Biol. 2024. PMID: 38275669 Free PMC article. Review.
-
Determination of benchmark doses for linear furanocoumarin consumption associated with inhibition of cytochrome P450 1A2 isoenzyme activity in healthy human adults.Toxicol Rep. 2021 Jul 22;8:1437-1444. doi: 10.1016/j.toxrep.2021.07.013. eCollection 2021. Toxicol Rep. 2021. PMID: 34377680 Free PMC article.
References
-
- Alehaideb Z., Sheriffdeen M., Law F. C. P. (2017). Furanocoumarin bioactives in the Apiaceae and Rutaceae families of plants. Canad. J. Pure Appl. Sci. 11, 4157–4167.
-
- Atsdr U. (2004). Guidance manual for the assessment of joint toxic action of chemical mixtures. Atlanta: US Department of Health and Human ServicesPublic Health Service, Agency for Toxic Substances and Disease Registry.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

