Cannabidiol Isolated From Cannabis sativa L. Protects Intestinal Barrier From In Vitro Inflammation and Oxidative Stress
- PMID: 33995048
- PMCID: PMC8115937
- DOI: 10.3389/fphar.2021.641210
Cannabidiol Isolated From Cannabis sativa L. Protects Intestinal Barrier From In Vitro Inflammation and Oxidative Stress
Abstract
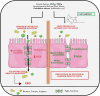
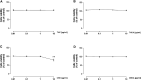
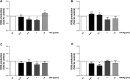
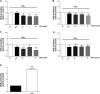
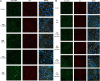
The relevance and incidence of intestinal bowel diseases (IBD) have been increasing over the last 50 years and the current therapies are characterized by severe side effects, making essential the development of new strategies that combine efficacy and safety in the management of human IBD. Herbal products are highly considered in research aimed at discovering new approaches for IBD therapy and, among others, Cannabis sativa L. has been traditionally used for centuries as an analgesic and anti-inflammatory remedy also in different gastrointestinal disorders. This study aims to investigate the effects of different C. sativa isolated compounds in an in vitro model of intestinal epithelium. The ability of treatments to modulate markers of intestinal dysfunctions was tested on Caco-2 intestinal cell monolayers. Our results, obtained by evaluation of ROS production, TEER and paracellular permeability measurements and tight junctions evaluation show Cannabidiol as the most promising compound against intestinal inflammatory condition. Cannabidiol is able to inhibit ROS production and restore epithelial permeability during inflammatory and oxidative stress conditions, suggesting its possible application as adjuvant in IBD management.
Keywords: Cannabis sativa; cannabidiol; intestinal barrier dysfunction; intestinal inflammation; intestinal permeability; transepithelial electrical resistance.
Copyright © 2021 Cocetta, Governa, Borgonetti, Tinazzi, Peron, Catanzaro, Berretta, Biagi, Manetti, Dall’Acqua and Montopoli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


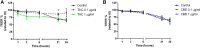
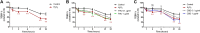

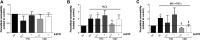
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

