The HCN Channel Blocker ZD7288 Induces Emesis in the Least Shrew (Cryptotis parva)
- PMID: 33995059
- PMCID: PMC8117105
- DOI: 10.3389/fphar.2021.647021
The HCN Channel Blocker ZD7288 Induces Emesis in the Least Shrew (Cryptotis parva)
Abstract
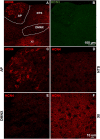
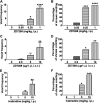
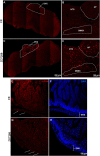
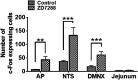
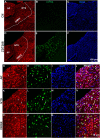
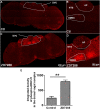
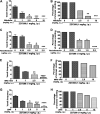
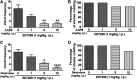
Subtypes (1-4) of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are widely expressed in the central and peripheral nervous systems, as well as the cells of smooth muscles in many organs. They mainly serve to regulate cellular excitability in these tissues. The HCN channel blocker ZD7288 has been shown to reduce apomorphine-induced conditioned taste aversion on saccharin preference in rats suggesting potential antinausea/antiemetic effects. Currently, in the least shew model of emesis we find that ZD7288 induces vomiting in a dose-dependent manner, with maximal efficacies of 100% at 1 mg/kg (i.p.) and 83.3% at 10 µg (i.c.v.). HCN channel subtype (1-4) expression was assessed using immunohistochemistry in the least shrew brainstem dorsal vagal complex (DVC) containing the emetic nuclei (area postrema (AP), nucleus tractus solitarius and dorsal motor nucleus of the vagus). Highly enriched HCN1 and HCN4 subtypes are present in the AP. A 1 mg/kg (i.p.) dose of ZD7288 strongly evoked c-Fos expression and ERK1/2 phosphorylation in the shrew brainstem DVC, but not in the in the enteric nervous system in the jejunum, suggesting a central contribution to the evoked vomiting. The ZD7288-evoked c-Fos expression exclusively occurred in tryptophan hydroxylase 2-positive serotonin neurons of the dorsal vagal complex, indicating activation of serotonin neurons may contribute to ZD7288-induced vomiting. To reveal its mechanism(s) of emetic action, we evaluated the efficacy of diverse antiemetics against ZD7288-evoked vomiting including the antagonists/inhibitors of: ERK1/2 (U0126), L-type Ca2+ channel (nifedipine); store-operated Ca2+ entry (MRS 1845); T-type Ca2+ channel (Z944), IP3R (2-APB), RyR receptor (dantrolene); the serotoninergic type 3 receptor (palonosetron); neurokinin 1 receptor (netupitant), dopamine type 2 receptor (sulpride), and the transient receptor potential vanilloid 1 receptor agonist, resiniferatoxin. All tested antiemetics except sulpride attenuated ZD7288-evoked vomiting to varying degrees. In sum, ZD7288 has emetic potential mainly via central mechanisms, a process which involves Ca2+ signaling and several emetic receptors. HCN channel blockers have been reported to have emetic potential in the clinic since they are currently used/investigated as therapeutic candidates for cancer therapy related- or unrelated-heart failure, pain, and cognitive impairment.
Keywords: HCN channel; ZD7288; calcium; emesis; emetic nuclei; least shrew.
Copyright © 2021 Zhong and Darmani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Thapsigargin-induced activation of Ca(2+)-CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew.Neuropharmacology. 2016 Apr;103:195-210. doi: 10.1016/j.neuropharm.2015.11.023. Epub 2015 Nov 26. Neuropharmacology. 2016. PMID: 26631534
-
Central and peripheral emetic loci contribute to vomiting evoked by the Akt inhibitor MK-2206 in the least shrew model of emesis.Eur J Pharmacol. 2021 Jun 5;900:174065. doi: 10.1016/j.ejphar.2021.174065. Epub 2021 Mar 26. Eur J Pharmacol. 2021. PMID: 33775646 Free PMC article.
-
Intracellular emetic signaling evoked by the L-type Ca2+ channel agonist FPL64176 in the least shrew (Cryptotis parva).Eur J Pharmacol. 2018 Sep 5;834:157-168. doi: 10.1016/j.ejphar.2018.06.035. Epub 2018 Jun 30. Eur J Pharmacol. 2018. PMID: 29966616 Free PMC article.
-
Ca2+ signaling and emesis: Recent progress and new perspectives.Auton Neurosci. 2017 Jan;202:18-27. doi: 10.1016/j.autneu.2016.07.006. Epub 2016 Jul 26. Auton Neurosci. 2017. PMID: 27473611 Review.
-
Central neurocircuitry associated with emesis.Am J Med. 2001 Dec 3;111 Suppl 8A:106S-112S. doi: 10.1016/s0002-9343(01)00849-x. Am J Med. 2001. PMID: 11749934 Review.
Cited by
-
Advances in research on the correlation between LTCCBs and cardiovascular diseases: A review.Medicine (Baltimore). 2025 Jun 20;104(25):e42799. doi: 10.1097/MD.0000000000042799. Medicine (Baltimore). 2025. PMID: 40550092 Free PMC article. Review.
-
Screening effects of HCN channel blockers on sleep/wake behavior in zebrafish.Front Neurosci. 2024 Mar 19;18:1375484. doi: 10.3389/fnins.2024.1375484. eCollection 2024. Front Neurosci. 2024. PMID: 38567282 Free PMC article.
-
A Comparative Study of the Antiemetic Effects of α2-Adrenergic Receptor Agonists Clonidine and Dexmedetomidine against Diverse Emetogens in the Least Shrew (Cryptotis parva) Model of Emesis.Int J Mol Sci. 2024 Apr 23;25(9):4603. doi: 10.3390/ijms25094603. Int J Mol Sci. 2024. PMID: 38731821 Free PMC article.
-
Moxibustion Regulates Gastrointestinal Motility via HCN1 in Functional Dyspepsia Rats.Med Sci Monit. 2021 Nov 30;27:e932885. doi: 10.12659/MSM.932885. Med Sci Monit. 2021. PMID: 34845181 Free PMC article.
References
-
- Adel N. (2017). Overview of Chemotherapy-Induced Nausea and Vomiting and Evidence-Based Therapies. Am. J. Manag. Care 23, S259–S265. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

