Role of Pharmacogenetics in Adverse Drug Reactions: An Update towards Personalized Medicine
- PMID: 33995067
- PMCID: PMC8120428
- DOI: 10.3389/fphar.2021.651720
Role of Pharmacogenetics in Adverse Drug Reactions: An Update towards Personalized Medicine
Abstract
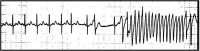
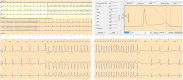
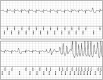
Adverse drug reactions (ADRs) are an important and frequent cause of morbidity and mortality. ADR can be related to a variety of drugs, including anticonvulsants, anaesthetics, antibiotics, antiretroviral, anticancer, and antiarrhythmics, and can involve every organ or apparatus. The causes of ADRs are still poorly understood due to their clinical heterogeneity and complexity. In this scenario, genetic predisposition toward ADRs is an emerging issue, not only in anticancer chemotherapy, but also in many other fields of medicine, including hemolytic anemia due to glucose-6-phosphate dehydrogenase (G6PD) deficiency, aplastic anemia, porphyria, malignant hyperthermia, epidermal tissue necrosis (Lyell's Syndrome and Stevens-Johnson Syndrome), epilepsy, thyroid diseases, diabetes, Long QT and Brugada Syndromes. The role of genetic mutations in the ADRs pathogenesis has been shown either for dose-dependent or for dose-independent reactions. In this review, we present an update of the genetic background of ADRs, with phenotypic manifestations involving blood, muscles, heart, thyroid, liver, and skin disorders. This review aims to illustrate the growing usefulness of genetics both to prevent ADRs and to optimize the safe therapeutic use of many common drugs. In this prospective, ADRs could become an untoward "stress test," leading to new diagnosis of genetic-determined diseases. Thus, the wider use of pharmacogenetic testing in the work-up of ADRs will lead to new clinical diagnosis of previously unsuspected diseases and to improved safety and efficacy of therapies. Improving the genotype-phenotype correlation through new lab techniques and implementation of artificial intelligence in the future may lead to personalized medicine, able to predict ADR and consequently to choose the appropriate compound and dosage for each patient.
Keywords: adverse drug reaction; brugada syndrome; diabetes; genetic test; long QT syndrome; personalized medicine; proarrhythmia; seizure.
Copyright © 2021 Micaglio, Locati, Monasky, Romani, Heilbron and Pappone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

