Systemic and Oral Immunogenicity of Porcine Epidemic Diarrhea Virus Antigen Fused to Poly-Fc of Immunoglobulin G and Expressed in ΔXT/FT Nicotiana benthamiana Plants
- PMID: 33995068
- PMCID: PMC8120289
- DOI: 10.3389/fphar.2021.653064
Systemic and Oral Immunogenicity of Porcine Epidemic Diarrhea Virus Antigen Fused to Poly-Fc of Immunoglobulin G and Expressed in ΔXT/FT Nicotiana benthamiana Plants
Abstract
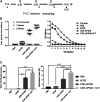
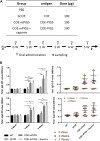
Porcine epidemic diarrhea virus (PEDV), a member of the Coronaviridae family has become increasingly probelmatic in the pig farming industry. Currently, there are no effective, globally applicable vaccines against PEDV. Here, we tested a recombinant PEDV vaccine candidate based on the expression of the core neutralising epitope (COE) of PEDV conjugated to polymeric immunoglobulin G scaffold (PIGS) in glycoengineered Nicotiana be nthamiana plants. The biological activity of COE-PIGS was demonstrated by binding to C1q component of the complement system, as well as the surface of antigen-presenting cells (APCs) in vitro. The recombinant COE-PIGS induced humoral and cellular immune responses specific for PEDV after both systemic and mucosal vaccination. Altogether, the data indicated that PEDV antigen fusion to poly-Fc could be a promising vaccine platform against respiratory PEDV infection.
Keywords: PEDV; immunity; mucosal; plant; vaccine.
Copyright © 2021 Tien, Yang, Jang, Kwon, Reljic and Kim.
Conflict of interest statement
Authors T-HK and M-SY were employed by the company Natural Bio-Materials Inc. (NBM). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


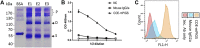
References
-
- Chang C. Y., Cheng I. C., Chang Y. C., Tsai P. S., Lai S. Y., Huang Y. L., et al. (2019). Identification of neutralizing monoclonal antibodies targeting novel conformational epitopes of the porcine epidemic diarrhoea virus spike protein. Sci. Rep. 9, 2529. 10.1038/s41598-019-39844-5 - DOI - PMC - PubMed
-
- Chang S. H., Bae J. L., Kang T. J., Kim J., Chung G. H., Lim C. W., et al. (2002). Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cell 14, 295–299. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

