CNS-Draining Meningeal Lymphatic Vasculature: Roles, Conundrums and Future Challenges
- PMID: 33995074
- PMCID: PMC8113819
- DOI: 10.3389/fphar.2021.655052
CNS-Draining Meningeal Lymphatic Vasculature: Roles, Conundrums and Future Challenges
Abstract
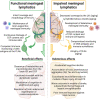
A genuine and functional lymphatic vascular system is found in the meninges that sheath the central nervous system (CNS). This unexpected (re)discovery led to a reevaluation of CNS fluid and solute drainage mechanisms, neuroimmune interactions and the involvement of meningeal lymphatics in the initiation and progression of neurological disorders. In this manuscript, we provide an overview of the development, morphology and unique functional features of meningeal lymphatics. An outline of the different factors that affect meningeal lymphatic function, such as growth factor signaling and aging, and their impact on the continuous drainage of brain-derived molecules and meningeal immune cells into the cervical lymph nodes is also provided. We also highlight the most recent discoveries about the roles of the CNS-draining lymphatic vasculature in different pathologies that have a strong neuroinflammatory component, including brain trauma, tumors, and aging-associated neurodegenerative diseases like Alzheimer's and Parkinson's. Lastly, we provide a critical appraisal of the conundrums, challenges and exciting questions involving the meningeal lymphatic system that ought to be investigated in years to come.
Keywords: aging; central nervous system; cerebrospinal fluid; disease; drainage; lymphatic vessels; meninges; neuroinflammation.
Copyright © 2021 das Neves, Delivanoglou and Da Mesquita.
Conflict of interest statement
SM is holding patents and patent applications related to the novel findings described in original manuscripts that are mentioned in this review article. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Achen M. G., Jeltsch M., Kukk E., Makinen T., Vitali A., Wilks A. F., et al. (1998). Vascular Endothelial Growth Factor D (VEGF-D) Is a Ligand for the Tyrosine Kinases VEGF Receptor 2 (Flk1) and VEGF Receptor 3 (Flt4). Proc. Natl. Acad. Sci. 95 (2), 548–553. 10.1073/pnas.95.2.548 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

