The Protective Effects of Sulforaphane on High-Fat Diet-Induced Obesity in Mice Through Browning of White Fat
- PMID: 33995092
- PMCID: PMC8116735
- DOI: 10.3389/fphar.2021.665894
The Protective Effects of Sulforaphane on High-Fat Diet-Induced Obesity in Mice Through Browning of White Fat
Abstract
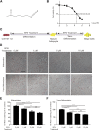
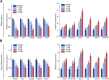
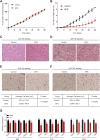
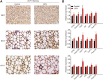
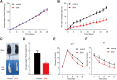
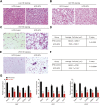
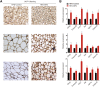
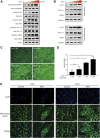
Background: Sulforaphane (SFN), an isothiocyanate naturally occurring in cruciferous vegetables, is a potent indirect antioxidant and a promising agent for the control of metabolic disorder disease. The glucose intolerance and adipogenesis induced by diet in rats was inhibited by SFN. Strategies aimed at induction of brown adipose tissue (BAT) could be a potentially useful way to against obesity. However, in vivo protective effect of SFN against obesity by browning white adipocyte has not been reported. Our present study is aimed at evaluation the efficacy of the SFN against the high-fat induced-obesity mice and investigating the potential mechanism. Methods: High-Fat Diet-induced obese female C57BL/6 mice were intraperitoneally injected with SFN (10 mg/kg) daily. Body weight was recorded every 3 days. 30 days later, glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed. At the end of experiment, fat mass were measured and the adipogenesis as well as browning associated genes expression in white adipose tissue (WAT) were determined by RT-qPCR and western blot. Histological examination of the adipose tissue samples were carried out with hematoxylin-eosin (HE) staining and immunofluorescence staining method. In vitro, pre-adipocytes C3H10T1/2 were treated with SFN to investigate the direct effects on adipogenesis. Results: SFN suppressed HFD-induced body weight gain and reduced the size of fat cells in mice. SFN suppressed the expression of key genes in adipogenesis, inhibited lipid accumulation in C3H10T1/2 cells, increased the expression of brown adipocyte-specific markers and mitochondrial biogenesis in vivo and in vitro, and decreased cellular and mitochondrial oxidative stress. These results suggested that SFN, as a nutritional factor, has great potential role in the battle against obesity by inducing the browning of white fat. Conclusion: SFN could significantly decrease the fat mass, and improve glucose metabolism and increase insulin sensitivity of HFD-induced obese mice by promoting the browning of white fat and enhancing the mitochondrial biogenesis in WAT. Our study proves that SFN could serve as a potential medicine in anti-obesity and related diseases.
Keywords: browning; high-fat diet; obesity; sulforaphane; white adipose browning.
Copyright © 2021 Liu, Fu, Chen, Luo, Zhu, Ji and Bian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Brunetta H. S., Politis-Barber V., Petrick H. L., Dennis K. M. J. H., Kirsh A. J., Barbeau P. A., et al. (2020). Nitrate Attenuates High Fat Diet-Induced Glucose Intolerance in Association with Reduced Epididymal Adipose Tissue Inflammation and Mitochondrial Reactive Oxygen Species Emission. J. Physiol. 598 (16), 3357–3371. 10.1113/jp279455 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

