Pseudoginsenoside F11 Enhances the Viability of Random-Pattern Skin Flaps by Promoting TFEB Nuclear Translocation Through AMPK-mTOR Signal Pathway
- PMID: 33995096
- PMCID: PMC8116945
- DOI: 10.3389/fphar.2021.667524
Pseudoginsenoside F11 Enhances the Viability of Random-Pattern Skin Flaps by Promoting TFEB Nuclear Translocation Through AMPK-mTOR Signal Pathway
Abstract
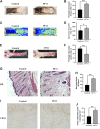
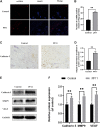
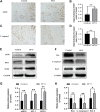
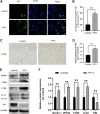
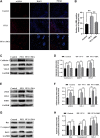
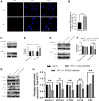
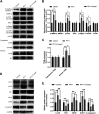
Random-pattern skin flap is widely used in tissue reconstruction. However, necrosis occurring in the distal part of the flap limits its clinical application to some extent. Activation of autophagy has been considered as an effective approach to enhance the survival of skin flaps. Pseudoginsenoside F11 (PF11), an ocotillol-type saponin, is an important component of Panax quinquefolium which has been shown to confer protection against cerebral ischemia and alleviate oxidative stress. However, it is currently unknown whether PF11 induces autophagy to improve the survival of skin flaps. In this study, we investigated the effects of PF11 on blood flow and tissue edema. The results of histological examination and western blotting showed that PF11 enhanced angiogenesis, alleviated apoptosis and oxidative stress, thereby improving the survival of the flap. Further experiments showed that PF11 promoted nuclear translocation of TFEB and by regulating the phosphorylation of AMPK. In summary, this study demonstrates that PF11 activates autophagy through the AMPK-TFEB signal pathway in skin flaps and it could be a promising strategy for enhancing flap viability.
Keywords: AMPK-mTOR signaling pathway; TFEB; angiogenesis; apoptosis; autophagy; oxidative stress; pseudoginsenoside F11; random-pattern skin flaps.
Copyright © 2021 Zhou, Zhang, Jiang, Li, Chen and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

