Protein Kinases Mediate Anti-Inflammatory Effects of Cannabidiol and Estradiol Against High Glucose in Cardiac Sodium Channels
- PMID: 33995099
- PMCID: PMC8115126
- DOI: 10.3389/fphar.2021.668657
Protein Kinases Mediate Anti-Inflammatory Effects of Cannabidiol and Estradiol Against High Glucose in Cardiac Sodium Channels
Abstract
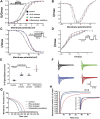
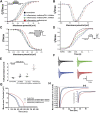
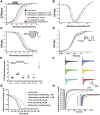
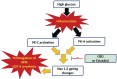
Background: Cardiovascular anomalies are predisposing factors for diabetes-induced morbidity and mortality. Recently, we showed that high glucose induces changes in the biophysical properties of the cardiac voltage-gated sodium channel (Nav1.5) that could be strongly correlated to diabetes-induced arrhythmia. However, the mechanisms underlying hyperglycemia-induced inflammation, and how inflammation provokes cardiac arrhythmia, are not well understood. We hypothesized that inflammation could mediate the high glucose-induced biophyscial changes on Nav1.5 through protein phosphorylation by protein kinases A and C. We also hypothesized that this signaling pathway is, at least partly, involved in the cardiprotective effects of cannabidiol (CBD) and 17β-estradiol (E2). Methods and Results: To test these ideas, we used Chinese hamster ovarian (CHO) cells transiently co-transfected with cDNA encoding human Nav1.5 α-subunit under control, a cocktail of inflammatory mediators or 100 mM glucose conditions (for 24 h). We used electrophysiological experiments and action potential modeling. Inflammatory mediators, similar to 100 mM glucose, right shifted the voltage dependence of conductance and steady-state fast inactivation and increased persistent current leading to computational prolongation of action potential (hyperexcitability) which could result in long QT3 arrhythmia. We also used human iCell cardiomyocytes derived from inducible pluripotent stem cells (iPSC-CMs) as a physiologically relevant system, and they replicated the effects produced by inflammatory mediators observed in CHO cells. In addition, activators of PK-A or PK-C replicated the inflammation-induced gating changes of Nav1.5. Inhibitors of PK-A or PK-C, CBD or E2 mitigated all the potentially deleterious effects provoked by high glucose/inflammation. Conclusion: These findings suggest that PK-A and PK-C may mediate the anti-inflammatory effects of CBD and E2 against high glucose-induced arrhythmia. CBD, via Nav1.5, may be a cardioprotective therapeutic approach in diabetic postmenopausal population.
Keywords: cannabidiol; diabetes; estradiol; high glucose; inflammation; protein kinase A; protein kinase C; sodium ion channels.
Copyright © 2021 Fouda and Ruben.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels.Br J Pharmacol. 2020 Jul;177(13):2932-2946. doi: 10.1111/bph.15020. Epub 2020 Mar 10. Br J Pharmacol. 2020. PMID: 32077098 Free PMC article.
-
Anti-inflammatory effects of cannabidiol against lipopolysaccharides in cardiac sodium channels.Br J Pharmacol. 2022 Dec;179(24):5259-5272. doi: 10.1111/bph.15936. Epub 2022 Aug 16. Br J Pharmacol. 2022. PMID: 35906756
-
Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol.Brain. 2016 Aug;139(Pt 8):2164-81. doi: 10.1093/brain/aww129. Epub 2016 Jun 5. Brain. 2016. PMID: 27267376 Free PMC article.
-
Regulation of Cardiac Voltage-Gated Sodium Channel by Kinases: Roles of Protein Kinases A and C.Handb Exp Pharmacol. 2018;246:161-184. doi: 10.1007/164_2017_53. Handb Exp Pharmacol. 2018. PMID: 29032483 Review.
-
Proton modulation of cardiac I Na: a potential arrhythmogenic trigger.Handb Exp Pharmacol. 2014;221:169-81. doi: 10.1007/978-3-642-41588-3_8. Handb Exp Pharmacol. 2014. PMID: 24737236 Review.
Cited by
-
The pharmacology and therapeutic role of cannabidiol in diabetes.Exploration (Beijing). 2023 Jul 12;3(5):20230047. doi: 10.1002/EXP.20230047. eCollection 2023 Oct. Exploration (Beijing). 2023. PMID: 37933286 Free PMC article.
-
Absolute Quantification of Nav1.5 Expression by Targeted Mass Spectrometry.Int J Mol Sci. 2022 Apr 10;23(8):4177. doi: 10.3390/ijms23084177. Int J Mol Sci. 2022. PMID: 35456996 Free PMC article.
-
Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle.Int J Mol Sci. 2023 Apr 26;24(9):7879. doi: 10.3390/ijms24097879. Int J Mol Sci. 2023. PMID: 37175587 Free PMC article. Review.
-
Lipopolysaccharide Modifies Sodium Current Kinetics through ROS and PKC Signalling in Induced Pluripotent Stem-Derived Cardiomyocytes from Brugada Syndrome Patient.J Cardiovasc Dev Dis. 2022 Apr 15;9(4):119. doi: 10.3390/jcdd9040119. J Cardiovasc Dev Dis. 2022. PMID: 35448095 Free PMC article.
-
Cannabidiol effects in stem cells: A systematic review.Biofactors. 2025 Jan-Feb;51(1):e2148. doi: 10.1002/biof.2148. Epub 2024 Dec 9. Biofactors. 2025. PMID: 39653426 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

