Assembly of Natively Synthesized Dual Chromophores Into Functional Actinorhodopsin
- PMID: 33995310
- PMCID: PMC8113403
- DOI: 10.3389/fmicb.2021.652328
Assembly of Natively Synthesized Dual Chromophores Into Functional Actinorhodopsin
Abstract
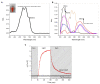
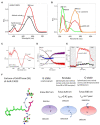
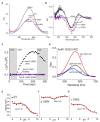
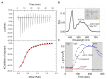
Microbial rhodopsin is a simple solar energy-capturing molecule compared to the complex photosynthesis apparatus. Light-driven proton pumping across the cell membrane is a crucial mechanism underlying microbial energy production. Actinobacteria is one of the highly abundant bacterial phyla in freshwater habitats, and members of this lineage are considered to boost heterotrophic growth via phototrophy, as indicated by the presence of actino-opsin (ActR) genes in their genome. However, it is difficult to validate their function under laboratory settings because Actinobacteria are not consistently cultivable. Based on the published genome sequence of Candidatus aquiluna sp. strain IMCC13023, actinorhodopsin from the strain (ActR-13023) was isolated and characterized in this study. Notably, ActR-13023 assembled with natively synthesized carotenoid/retinal (used as a dual chromophore) and functioned as a light-driven outward proton pump. The ActR-13023 gene and putative genes involved in the chromophore (retinal/carotenoid) biosynthetic pathway were detected in the genome, indicating the functional expression ActR-13023 under natural conditions for the utilization of solar energy for proton translocation. Heterologous expressed ActR-13023 exhibited maximum absorption at 565 nm with practical proton pumping ability. Purified ActR-13023 could be reconstituted with actinobacterial carotenoids for additional light-harvesting. The existence of actinorhodopsin and its chromophore synthesis machinery in Actinobacteria indicates the inherent photo-energy conversion function of this microorganism. The assembly of ActR-13023 to its synthesized chromophores validated the microbial community's importance in the energy cycle.
Keywords: actinobacteria; carotenoid; dual chromophore actinorhodopsin; light-driven proton pumps; microbial rhodopsin; retinal.
Copyright © 2021 Chuon, Kim, Meas, Shim, Cho, Kang, Kim, Cho and Jung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
acI Actinobacteria Assemble a Functional Actinorhodopsin with Natively Synthesized Retinal.Appl Environ Microbiol. 2018 Nov 30;84(24):e01678-18. doi: 10.1128/AEM.01678-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30315080 Free PMC article.
-
Photochemical characterization of actinorhodopsin and its functional existence in the natural host.Biochim Biophys Acta. 2016 Dec;1857(12):1900-1908. doi: 10.1016/j.bbabio.2016.09.006. Epub 2016 Sep 20. Biochim Biophys Acta. 2016. PMID: 27659506
-
Characterization of an Unconventional Rhodopsin from the Freshwater Actinobacterium Rhodoluna lacicola.J Bacteriol. 2015 Aug;197(16):2704-12. doi: 10.1128/JB.00386-15. Epub 2015 Jun 8. J Bacteriol. 2015. PMID: 26055118 Free PMC article.
-
Omega Rhodopsins: A Versatile Class of Microbial Rhodopsins.J Microbiol Biotechnol. 2020 May 28;30(5):633-641. doi: 10.4014/jmb.1912.12010. J Microbiol Biotechnol. 2020. PMID: 32482928 Free PMC article. Review.
-
Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology.Microbiol Mol Biol Rev. 2016 Sep 14;80(4):929-54. doi: 10.1128/MMBR.00003-16. Print 2016 Dec. Microbiol Mol Biol Rev. 2016. PMID: 27630250 Free PMC article. Review.
Cited by
-
Carotenoid binding in Gloeobacteria rhodopsin provides insights into divergent evolution of xanthorhodopsin types.Commun Biol. 2022 May 30;5(1):512. doi: 10.1038/s42003-022-03429-2. Commun Biol. 2022. PMID: 35637261 Free PMC article.
-
Genome editing in ubiquitous freshwater Actinobacteria.Appl Environ Microbiol. 2024 Nov 20;90(11):e0086524. doi: 10.1128/aem.00865-24. Epub 2024 Oct 16. Appl Environ Microbiol. 2024. PMID: 39412376 Free PMC article.
-
Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering.Front Chem. 2022 Jun 22;10:879609. doi: 10.3389/fchem.2022.879609. eCollection 2022. Front Chem. 2022. PMID: 35815212 Free PMC article. Review.
-
Proton-pumping photoreceptor controls expression of ABC transporter by regulating transcription factor through light.Commun Biol. 2024 Jun 29;7(1):789. doi: 10.1038/s42003-024-06471-4. Commun Biol. 2024. PMID: 38951607 Free PMC article.
-
Recent Differentiation of Aquatic Bacterial Communities in a Hydrological System in the Cuatro Ciénegas Basin, After a Natural Perturbation.Front Microbiol. 2022 Apr 28;13:825167. doi: 10.3389/fmicb.2022.825167. eCollection 2022. Front Microbiol. 2022. PMID: 35572686 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

