Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice
- PMID: 33995345
- PMCID: PMC8118162
- DOI: 10.3389/fimmu.2021.616394
Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice
Abstract
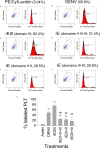
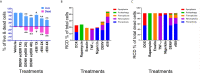
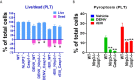
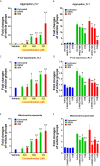
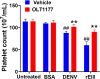
In tropical and subtropical regions, mosquito-borne dengue virus (DENV) infections can lead to severe dengue, also known as dengue hemorrhage fever, which causes bleeding, thrombocytopenia, and blood plasma leakage and increases mortality. Although DENV-induced platelet cell death was linked to disease severity, the role of responsible viral factors and the elicitation mechanism of abnormal platelet activation and cell death remain unclear. DENV and virion-surface envelope protein domain III (EIII), a cellular binding moiety of the virus particle, highly increase during the viremia stage. Our previous report suggested that exposure to such viremia EIII levels can lead to cell death of endothelial cells, neutrophils, and megakaryocytes. Here we found that both DENV and EIII could induce abnormal platelet activation and predominantly necrotic cell death pyroptosis. Blockages of EIII-induced platelet signaling using the competitive inhibitor chondroitin sulfate B or selective Nlrp3 inflammasome inhibitors OLT1177 and Z-WHED-FMK markedly ameliorated DENV- and EIII-induced thrombocytopenia, platelet activation, and cell death. These results suggest that EIII could be considered as a virulence factor of DENV, and that Nlrp3 inflammasome is a feasible target for developing therapeutic approaches against dengue-induced platelet defects.
Keywords: Nlrp3 inflammasome; apoptosis; dengue hemorrhage fever; envelope protein domain III; ferroptosis; necroptosis; platelet; pyroptosis.
Copyright © 2021 Lien, Chan, Sun, Wu, Lin, Lin and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dengue Envelope Protein as a Cytotoxic Factor Inducing Hemorrhage and Endothelial Cell Death in Mice.Int J Mol Sci. 2024 Oct 9;25(19):10858. doi: 10.3390/ijms251910858. Int J Mol Sci. 2024. PMID: 39409186 Free PMC article.
-
Exposure to Dengue Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Endothelial Dysfunction and Hemorrhage in Mice.Front Immunol. 2021 Feb 25;12:617251. doi: 10.3389/fimmu.2021.617251. eCollection 2021. Front Immunol. 2021. PMID: 33717109 Free PMC article.
-
Dengue Virus Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent NETosis-Mediated Inflammation in Mice.Front Immunol. 2021 Mar 17;12:618577. doi: 10.3389/fimmu.2021.618577. eCollection 2021. Front Immunol. 2021. PMID: 33815373 Free PMC article.
-
Inflammasome Fuels Dengue Severity.Front Cell Infect Microbiol. 2020 Sep 10;10:489. doi: 10.3389/fcimb.2020.00489. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33014899 Free PMC article. Review.
-
Dengue Virus and Platelets: From the Biology to the Clinic.Viral Immunol. 2022 Jun;35(5):349-358. doi: 10.1089/vim.2021.0135. Epub 2022 Apr 28. Viral Immunol. 2022. PMID: 35483090 Review.
Cited by
-
Inflammasome activation by viral infection: mechanisms of activation and regulation.Front Microbiol. 2023 Aug 7;14:1247377. doi: 10.3389/fmicb.2023.1247377. eCollection 2023. Front Microbiol. 2023. PMID: 37608944 Free PMC article. Review.
-
Activating SRC/MAPK signaling via 5-HT1A receptor contributes to the effect of vilazodone on improving thrombocytopenia.Elife. 2024 Apr 4;13:RP94765. doi: 10.7554/eLife.94765. Elife. 2024. PMID: 38573820 Free PMC article.
-
Simulation of Hemorrhage Pathogenesis in Mice through Dual Stimulation with Dengue Envelope Protein Domain III-Coated Nanoparticles and Antiplatelet Antibody.Int J Mol Sci. 2023 May 25;24(11):9270. doi: 10.3390/ijms24119270. Int J Mol Sci. 2023. PMID: 37298220 Free PMC article.
-
Dengue Envelope Protein as a Cytotoxic Factor Inducing Hemorrhage and Endothelial Cell Death in Mice.Int J Mol Sci. 2024 Oct 9;25(19):10858. doi: 10.3390/ijms251910858. Int J Mol Sci. 2024. PMID: 39409186 Free PMC article.
-
Aedes aegypti saliva modulates inflammasome activation and facilitates flavivirus infection in vitro.iScience. 2023 Dec 2;27(1):108620. doi: 10.1016/j.isci.2023.108620. eCollection 2024 Jan 19. iScience. 2023. PMID: 38188518 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

