Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by the Interplay Between ORAI1 and InsP3 Receptors
- PMID: 33995380
- PMCID: PMC8113648
- DOI: 10.3389/fimmu.2021.659790
Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by the Interplay Between ORAI1 and InsP3 Receptors
Abstract
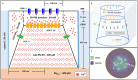
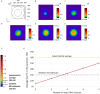
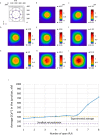
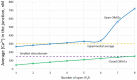
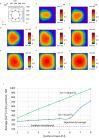
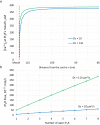
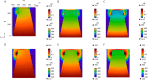
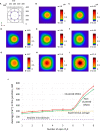
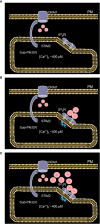
Ca2+ signaling plays an essential role in T cell activation, which is a key step to start an adaptive immune response. During the transition from a quiescent to a fully activated state, Ca2+ microdomains characterized by reduced spatial and temporal extents are observed in the junctions between the plasma membrane (PM) and the endoplasmic reticulum (ER). Such Ca2+ responses can also occur in response to T cell adhesion to other cells or extracellular matrix proteins in otherwise unstimulated T cells. These non-TCR/CD3-dependent Ca2+ microdomains rely on d-myo-inositol 1,4,5-trisphosphate (IP3) signaling and subsequent store operated Ca2+ entry (SOCE) via the ORAI/STIM system. The detailed molecular mechanism of adhesion-dependent Ca2+ microdomain formation remains to be fully elucidated. We used mathematical modeling to investigate the spatiotemporal characteristics of T cell Ca2+ microdomains and their molecular regulators. We developed a reaction-diffusion model using COMSOL Multiphysics to describe the evolution of cytosolic and ER Ca2+ concentrations in a three-dimensional ER-PM junction. Equations are based on a previously proposed realistic description of the junction, which is extended to take into account IP3 receptors (IP3R) that are located next to the junction. The first model only considered the ORAI channels and the SERCA pumps. Taking into account the existence of preformed clusters of ORAI1 and STIM2, ORAI1 slightly opens in conditions of a full ER. These simulated Ca2+ microdomains are too small as compared to those observed in unstimulated T cells. When considering the opening of the IP3Rs located near the junction, the local depletion of ER Ca2+ allows for larger Ca2+ fluxes through the ORAI1 channels and hence larger local Ca2+ concentrations. Computational results moreover show that Ca2+ diffusion in the ER has a major impact on the Ca2+ changes in the junction, by affecting the local Ca2+ gradients in the sub-PM ER. Besides pointing out the likely involvement of the spontaneous openings of IP3Rs in the activation of SOCE in conditions of T cell adhesion prior to full activation, the model provides a tool to investigate how Ca2+ microdomains extent and interact in response to T cell receptor activation.
Keywords: COMSOL; Calcium Signaling, T-cells; Store operated calcium entry (SOCE); computational model; non-TCR/CD3-dependent microdomains.
Copyright © 2021 Gil, Guse and Dupont.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

