Early Emergence of Adaptive Mechanisms Sustaining Ig Production: Application to Antibody Therapy
- PMID: 33995412
- PMCID: PMC8117215
- DOI: 10.3389/fimmu.2021.671998
Early Emergence of Adaptive Mechanisms Sustaining Ig Production: Application to Antibody Therapy
Abstract
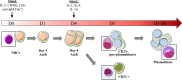
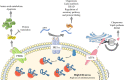
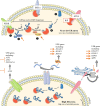
Antibody therapy, where artificially-produced immunoglobulins (Ig) are used to treat pathological conditions such as auto-immune diseases and cancers, is a very innovative and competitive field. Although substantial efforts have been made in recent years to obtain specific and efficient antibodies, there is still room for improvement especially when considering a precise tissular targeting or increasing antigen affinity. A better understanding of the cellular and molecular steps of terminal B cell differentiation, in which an antigen-activated B cell becomes an antibody secreting cell, may improve antibody therapy. In this review, we use our recently published data about human B cell differentiation, to show that the mechanisms necessary to adapt a metamorphosing B cell to its new secretory function appear quite early in the differentiation process i.e., at the pre-plasmablast stage. After characterizing the molecular pathways appearing at this stage, we will focus on recent findings about two main processes involved in antibody production: unfolded protein response (UPR) and endoplasmic reticulum (ER) stress. We'll show that many genes coding for factors involved in UPR and ER stress are induced at the pre-plasmablast stage, sustaining our hypothesis. Finally, we propose to use this recently acquired knowledge to improve productivity of industrialized therapeutic antibodies.
Keywords: B cell differentiation; ER stress; RNA-seq; UPR; mAbs.
Copyright © 2021 Lemarié, Chatonnet, Caron and Fest.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

