Aging-Induced Dysbiosis of Gut Microbiota as a Risk Factor for Increased Listeria monocytogenes Infection
- PMID: 33995413
- PMCID: PMC8115019
- DOI: 10.3389/fimmu.2021.672353
Aging-Induced Dysbiosis of Gut Microbiota as a Risk Factor for Increased Listeria monocytogenes Infection
Abstract
Invasive foodborne Listeria monocytogenes infection causes gastroenteritis, septicemia, meningitis, and chorioamnionitis, and is associated with high case-fatality rates in the elderly. It is unclear how aging alters gut microbiota, increases risk of listeriosis, and causes dysbiosis post-infection. We used a geriatric murine model of listeriosis as human surrogate of listeriosis for aging individuals to study the effect of aging and L. monocytogenes infection. Aging and listeriosis-induced perturbation of gut microbiota and disease severity were compared between young-adult and old mice. Young-adult and old mice were dosed intragastrically with L. monocytogenes. Fecal pellets were collected pre- and post-infection for microbiome analysis. Infected old mice had higher Listeria colonization in liver, spleen, and feces. Metagenomics analyses of fecal DNA-sequences showed increase in α-diversity as mice aged, and infection reduced its diversity. The relative abundance of major bacterial phylum like, Bacteroidetes and Firmicutes remained stable over aging or infection, while the Verrucomicrobia phylum was significantly reduced only in infected old mice. Old mice showed a marked reduction in Clostridaiceae and Lactobacillaceae bacteria even before infection when compared to uninfected young-adult mice. L. monocytogenes infection increased the abundance of Porphyromonadaceae and Prevotellaceae in young-adult mice, while members of the Ruminococcaceae and Lachnospiraceae family were significantly increased in old mice. The abundance of the genera Blautia and Alistipes were significantly reduced post-infection in young-adult and in old mice as compared to their uninfected counterparts. Butyrate producing, immune-modulating bacterial species, like Pseudoflavonifractor and Faecalibacterium were significantly increased only in old infected mice, correlating with increased intestinal inflammatory mRNA up-regulation from old mice tissue. Histologic analyses of gastric tissues showed extensive lesions in the Listeria-infected old mice, more so in the non-glandular region and fundus than in the pylorus. Commensal species like Lactobacillus, Clostridiales, and Akkermansia were only abundant in infected young-adult mice but their abundance diminished in the infected old mice. Listeriosis in old mice enhances the abundance of butyrate-producing inflammatory members of the Ruminococcaceae/Lachnospiraceae bacteria while reducing/eliminating beneficial commensals in the gut. Results of this study indicate that, aging may affect the composition of gut microbiota and increase the risk of invasive L. monocytogenes infection.
Keywords: Listeria monocytogenes; aging; dysbiosis; gut microbiota; inflammation; listeriosis; metagenomics.
Copyright © 2021 Alam, Gangiredla, Hasan, Barnaba and Tartera.
Conflict of interest statement
Author NH was employed by the company CosmosID. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
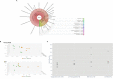

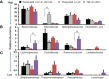




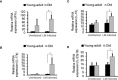
References
-
- Pohl AM, Pouillot R, Bazaco MC, Wolpert BJ, Healy JM, Bruce BB, et al. . Differences Among Incidence Rates of Invasive Listeriosis in the U.S. Foodnet Population by Age, Sex, Race/Ethnicity, and Pregnancy Status, 2008-2016. Foodborne Pathog Dis (2019) 16(4):290–7. 10.1089/fpd.2018.2548 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

