Proteomics, toxicity and antivenom neutralization of Sri Lankan and Indian Russell's viper (Daboia russelii) venoms
- PMID: 33995514
- PMCID: PMC8092856
- DOI: 10.1590/1678-9199-JVATITD-2020-0177
Proteomics, toxicity and antivenom neutralization of Sri Lankan and Indian Russell's viper (Daboia russelii) venoms
Abstract
Background: The western Russell's viper (Daboia russelii) is widely distributed in South Asia, and geographical venom variation is anticipated among distant populations. Antivenoms used for Russell's viper envenomation are, however, raised typically against snakes from Southern India. The present study investigated and compared the venom proteomes of D. russelii from Sri Lanka (DrSL) and India (DrI), the immunorecognition of Indian VINS Polyvalent Antivenom (VPAV) and its efficacy in neutralizing the venom toxicity.
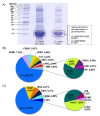
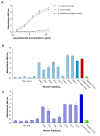
Methods: The venoms of DrSL and DrI were decomplexed with C18 high-performance liquid chromatography and SDS-polyacrylamide gel electrophoresis under reducing conditions. The proteins fractionated were identified through nano-ESI-liquid chromatography-tandem mass spectrometry (LCMS/MS). The immunological studies were conducted with enzyme-linked immunosorbent assay. The neutralization of the venom procoagulant effect was evaluated in citrated human plasma. The neutralization of the venom lethality was assessed in vivo in mice adopting the WHO protocol.
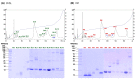
Results: DrSL and DrI venom proteomes showed comparable major protein families, with phospholipases A2 (PLA2) being the most abundant (> 60% of total venom proteins) and diverse (six protein forms identified). Both venoms were highly procoagulant and lethal (intravenous median lethal dose in mice, LD50 = 0.24 and 0.32 µg/g, for DrSL and DrI, respectively), while lacking hemorrhagic and anticoagulant activities. VPAV was immunoreactive toward DrSL and DrI venoms, indicating conserved protein antigenicity in the venoms. The high molecular weight venom proteins were, however, more effectively immunorecognized than small ones. VPAV was able to neutralize the coagulopathic and lethal effects of the venoms moderately.
Conclusion: Considering that a large amount of venom can be injected by Russell's viper during envenomation, the potency of antivenom can be further improved for optimal neutralization and effective treatment. Region-specific venoms and key toxins may be incorporated into the immunization procedure during antivenom production.
Keywords: Antivenom potency; Antivenomics; Geographical variation; Venomics.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Proteomics, functional characterization and antivenom neutralization of the venom of Pakistani Russell's viper (Daboia russelii) from the wild.J Proteomics. 2018 Jul 15;183:1-13. doi: 10.1016/j.jprot.2018.05.003. Epub 2018 May 3. J Proteomics. 2018. PMID: 29729992
-
Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka.J Proteomics. 2019 Sep 15;207:103443. doi: 10.1016/j.jprot.2019.103443. Epub 2019 Jul 17. J Proteomics. 2019. PMID: 31325606
-
Immunoreactivity and neutralization efficacy of Pakistani Viper Antivenom (PVAV) against venoms of Saw-scaled Vipers (Echis carinatus subspp.) and Western Russell's Vipers (Daboia russelii) from the Indian subcontinent.Acta Trop. 2024 Feb;250:107099. doi: 10.1016/j.actatropica.2023.107099. Epub 2023 Dec 12. Acta Trop. 2024. PMID: 38097152
-
Proteomic analysis reveals geographic variation in venom composition of Russell's Viper in the Indian subcontinent: implications for clinical manifestations post-envenomation and antivenom treatment.Expert Rev Proteomics. 2018 Oct;15(10):837-849. doi: 10.1080/14789450.2018.1528150. Expert Rev Proteomics. 2018. PMID: 30247947 Review.
-
Proteomic diversity of Russell's viper venom: exploring PLA2 isoforms, pharmacological effects, and inhibitory approaches.Arch Toxicol. 2024 Nov;98(11):3569-3584. doi: 10.1007/s00204-024-03849-5. Epub 2024 Aug 24. Arch Toxicol. 2024. PMID: 39181947 Free PMC article. Review.
Cited by
-
From birth to bite: the evolutionary ecology of India's medically most important snake venoms.BMC Biol. 2024 Jul 29;22(1):161. doi: 10.1186/s12915-024-01960-8. BMC Biol. 2024. PMID: 39075553 Free PMC article.
-
Evaluation of the properties of Bungarus caeruleus venom and checking the efficacy of antivenom used in Bangladesh for its bite treatment.Toxicon X. 2023 Jan 3;17:100149. doi: 10.1016/j.toxcx.2023.100149. eCollection 2023 Mar. Toxicon X. 2023. PMID: 36654657 Free PMC article.
-
Biochemical and proteomic analyses of venom from a new pit viper, Protobothrops kelomohy.J Venom Anim Toxins Incl Trop Dis. 2022 Apr 11;28:e20210080. doi: 10.1590/1678-9199-JVATITD-2021-0080. eCollection 2022. J Venom Anim Toxins Incl Trop Dis. 2022. PMID: 35432492 Free PMC article.
-
Neutrophil Gelatinase-Associated Lipocalin Acts as a Robust Early Diagnostic Marker for Renal Replacement Therapy in Patients with Russell's Viper Bite-Induced Acute Kidney Injuries.Toxins (Basel). 2021 Nov 12;13(11):797. doi: 10.3390/toxins13110797. Toxins (Basel). 2021. PMID: 34822581 Free PMC article.
-
The Need for Next-Generation Antivenom for Snakebite Envenomation in India.Toxins (Basel). 2023 Aug 18;15(8):510. doi: 10.3390/toxins15080510. Toxins (Basel). 2023. PMID: 37624267 Free PMC article. Review.
References
-
- Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:17063. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
