Cost-Effectiveness and Budget Impact Analyses of Pneumococcal Vaccination in Indonesia
- PMID: 33995536
- PMCID: PMC8096558
- DOI: 10.1155/2021/7494965
Cost-Effectiveness and Budget Impact Analyses of Pneumococcal Vaccination in Indonesia
Abstract
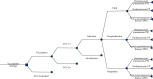
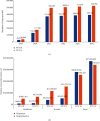
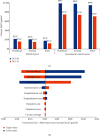
As a country with the high number of deaths due to pneumococcal disease, Indonesia has not yet included pneumococcal vaccination into the routine program. This study aimed to analyse the cost-effectiveness and the budget impact of pneumococcal vaccination in Indonesia by developing an age-structured cohort model. In a comparison with no vaccination, the use of two vaccines (PCV10 and PCV13) within two pricing scenarios (UNICEF and government contract price) was taken into account. To estimate the cost-effectiveness value, a 5-year time horizon was applied by extrapolating the outcome of the individual in the modelled cohort until 5 years of age with a 1-month analytical cycle. To estimate the affordability value, a 6-year period (2019-2024) was applied by considering the government's strategic plan on pneumococcal vaccination. In a comparison with no vaccination, the results showed that vaccination would reduce pneumococcal disease by 1,702,548 and 2,268,411 cases when using PCV10 and PCV13, respectively. Vaccination could potentially reduce the highest treatment cost from the payer perspective at $53.6 million and $71.4 million for PCV10 and PCV13, respectively. Applying the UNICEF price, the incremental cost-effectiveness ratio (ICER) from the healthcare perspective would be $218 and $162 per QALY-gained for PCV10 and PCV13, respectively. Applying the government contract price, the ICER would be $987 and $747 per QALY-gained for PCV10 and PCV13, respectively. The result confirmed that PCV13 was more cost-effective than PCV10 with both prices. In particular, introduction cost per child was estimated to be $0.91 and vaccination cost of PCV13 per child (3 doses) was estimated to be $16.61 and $59.54 with UNICEF and government contract prices, respectively. Implementation of nationwide vaccination would require approximately $73.3-$75.0 million (13-14% of routine immunization budget) and $257.4-$263.5 million (45-50% of routine immunization budget) with UNICEF and government contract prices, respectively. Sensitivity analysis showed that vaccine efficacy, mortality rate, and vaccine price were the most influential parameters affecting the ICER. In conclusion, pneumococcal vaccination would be a highly cost-effective intervention to be implemented in Indonesia. Yet, applying PCV13 with UNICEF price would give the best cost-effectiveness and affordability values on the routine immunization budget.
Copyright © 2021 Auliya A. Suwantika et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures
Similar articles
-
Do Pneumococcal Conjugate Vaccines Represent Good Value for Money in a Lower-Middle Income Country? A Cost-Utility Analysis in the Philippines.PLoS One. 2015 Jul 1;10(7):e0131156. doi: 10.1371/journal.pone.0131156. eCollection 2015. PLoS One. 2015. PMID: 26131961 Free PMC article.
-
Cost-effectiveness analysis of pneumococcal conjugate vaccine introduction in Paraguay.Vaccine. 2015 May 7;33 Suppl 1:A143-53. doi: 10.1016/j.vaccine.2014.12.078. Vaccine. 2015. PMID: 25919155
-
An updated cost-effectiveness analysis of pneumococcal conjugate vaccine among children in Thailand.Vaccine. 2019 Jul 26;37(32):4551-4560. doi: 10.1016/j.vaccine.2019.06.015. Epub 2019 Jul 4. Vaccine. 2019. PMID: 31280944
-
Impact of Switch Options on the Economics of Pneumococcal Conjugate Vaccine (PCV) Introduction in Indonesia.Vaccines (Basel). 2020 May 18;8(2):233. doi: 10.3390/vaccines8020233. Vaccines (Basel). 2020. PMID: 32443523 Free PMC article. Review.
-
Cost Effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: Systematic Literature Review with Focus on Methods and Assumptions.Pharmacoeconomics. 2019 Sep;37(9):1093-1127. doi: 10.1007/s40273-019-00805-5. Pharmacoeconomics. 2019. PMID: 31025189
Cited by
-
Accelerating Pneumococcal Conjugate Vaccine introductions in Indonesia: key learnings from 2017 to 2022.Infect Dis Poverty. 2023 Nov 28;12(1):107. doi: 10.1186/s40249-023-01161-5. Infect Dis Poverty. 2023. PMID: 38017524 Free PMC article.
-
Epidemiology, Nasopharyngeal Carriage, Serotype Prevalence, and Antibiotic Resistance of Streptococcus pneumoniae in Indonesia.Infect Dis Ther. 2020 Dec;9(4):723-736. doi: 10.1007/s40121-020-00330-5. Epub 2020 Aug 30. Infect Dis Ther. 2020. PMID: 32864725 Free PMC article. Review.
-
Burden of Pneumococcal Disease in Young Children Due to Serotypes Contained in Different Pneumococcal Conjugate Vaccines in Eight Asian Countries and Territories.Vaccines (Basel). 2024 Oct 19;12(10):1197. doi: 10.3390/vaccines12101197. Vaccines (Basel). 2024. PMID: 39460362 Free PMC article.
References
-
- WHO. Pneumonia fact sheet. http://www.who.int/mediacentre/factsheets/fs331/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

