Novel trends with biologics in inflammatory bowel disease: sequential and combined approaches
- PMID: 33995579
- PMCID: PMC8082976
- DOI: 10.1177/17562848211006669
Novel trends with biologics in inflammatory bowel disease: sequential and combined approaches
Abstract
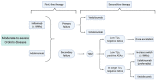
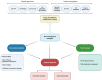
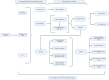
Inflammatory bowel disease (IBD) management has changed dramatically over the past 20 years, after the introduction of targeted biological therapies. However, the impact of these new drugs in changing the natural history of disease is still under debate. Recent evidence seems to suggest that the extent of their efficacy might be, at least partially, dependent on the timing of their introduction and on the subsequent management strategy. In this complex landscape, the potential role for a more dynamic approach with treatments based on sequencing and combining targeted therapies has been explored only minimally so far. In this review, we aim to explore the potential biological rationale behind the use of sequential and combination therapies in IBD, to summarise the current knowledge on this topic and to propose a management algorithm that combines these notions.
Keywords: Crohn’s disease; combination therapy; dual targeted therapy; swap; switch; switch;; ulcerative colitis, ;.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare the following conflicts of interest: Giuseppe Privitera received consultancy fees from Alphasigma. Daniela Pugliese received speaker fees from AbbVie, MSD, Takeda, Janssen and Pfizer. Franco Scaldaferri: advisory board for Abbvie, Janssen, MSD, Sanofi and Takeda. Luisa Guidi: consultancies and/or speaker fees from: AbbVie, Janssen, MSD, Mundipharma, Takeda, Vifor Pharma and Zambon. Antonio Gasbarrini reports personal fees for consultancy for Eisai S.r.l., 3PSolutions, Real Time Meeting, Fondazione Istituto Danone, Sinergie S.r.l. Board MRGE, and Sanofi S.p.A, personal fees for acting as a speaker for Takeda S.p.A, AbbVie, and Sandoz S.p.A, and personal fees for acting on advisory boards for VSL3 and Eisai. Alessandro Armuzzi: consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, MSD, Mylan, Pfizer, Samsung Bioepis, Sandoz and Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Biogen, Ferring, Giliead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Novartis, Pfizer, Sandoz, Samsung Bioepis and Takeda; and research grants from MSD, Pfizer and Takeda. All the other authors have no conflict of interest to declare.
Figures

References
-
- Verdon C, Reinglas J, Coulombe J, et al. No change in surgical and hospitalization trends despite higher exposure to anti-tumor necrosis factor in inflammatory bowel disease in the Québec Provincial Database from 1996 to 2015. Inflamm Bowel Dis. Epub ahead of print 17 July 2020. DOI: 10.1093/ibd/izaa166. - DOI - PubMed
-
- Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2019; 68: 423–433. - PubMed
-
- Targownik LE, Tennakoon A, Leung S, et al. Temporal trends in initiation of therapy with tumor necrosis factor antagonists for patients with inflammatory bowel disease: a population-based analysis. Clin Gastroenterol Hepatol 2017; 15: 1061–1070.e1. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

