Lenvatinib induces anticancer activity in gallbladder cancer by targeting AKT
- PMID: 33995632
- PMCID: PMC8120192
- DOI: 10.7150/jca.50292
Lenvatinib induces anticancer activity in gallbladder cancer by targeting AKT
Abstract
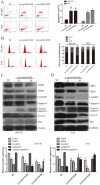
Gallbladder cancer (GBC) is characterized by poor prognosis, early metastasis, and high recurrence rates, which seriously threaten human health. The effect of lenvatinib, a widely used drug in anti-hepatocellular carcinoma in China, on GBC progress, as well as its underlying molecular mechanism, remains unclear. Therefore, the present study investigated the effect of lenvatinib on human GBC GBC-SD and NOZ cells and its underlying mechanisms. A series of experiments, including cell proliferation, clone formation, wound healing, and cell migration and invasion assays, as well as flow cytometry, were performed to investigate the anticancer effect of lenvatinib on GBC. Western blotting was used to detect alterations in protein expression of CKD2, CKD4, cyclin D1, caspase-9, matrix metalloproteinase (MMP)-2, cell migration-inducing protein (CEMIP) and phospho-AKT (p-AKT). In addition, the chemosensitivity of lenvatinib-treated GBC cells to gemcitabine (GEM) and whether the activation of phosphoinositide 3 kinase (PI3K)/AKT contributed to the chemoresistance were determined. Finally, the anticancer effect of lenvatinib in vivo was detected using a xenograft mouse model. These data showed that treatment with lenvatinib inhibited cell proliferation, colony formation ability, migration, induced apoptosis, regulated cell cycle and resulted in decreased resistance to GEM. Treatment with lenvatinib decreased the expression of MMP-2, CEMIP, CDK2, CDK4 and cyclin D1, and increased the expression of cleaved caspase-9, which was mediated by the inactivation of the PI3K/AKT pathway in vitro. In addition, lenvatinib inhibited autophagy in GBC-SD and NOZ cells. Besides, Lenvatinib suppressed GBC cell growth in vivo by targeting p-AKT. In combination, the present data indicated that lenvatinib plays a potential anticancer role in GBC by downregulating the expression of p-AKT.
Keywords: AKT; apoptosis; gallbladder cancer; lenvatinib; migration; proliferation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Fukudo M, Ito T, Mizuno T, Shinsako K, Hatano E, Uemoto S. et al. Exposure-toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clinical pharmacokinetics. 2014;53:185–96. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology. 2009;10:25–34. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF. et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359:378–90. - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

