Cardamonin inhibits the progression of oesophageal cancer by inhibiting the PI3K/AKT signalling pathway
- PMID: 33995637
- PMCID: PMC8120183
- DOI: 10.7150/jca.55519
Cardamonin inhibits the progression of oesophageal cancer by inhibiting the PI3K/AKT signalling pathway
Abstract
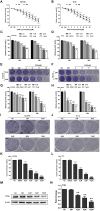
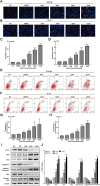
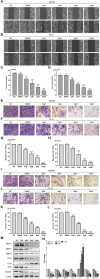
Background: Oesophageal cancer is the most common malignant tumour with a poor prognosis, and the current treatment methods are limited. Therefore, identifying effective treatment methods has become a research hotspot. Cardamonin (CAR) is a natural chalcone compound and has been reported to play an anticancer role in several cancers. However, its function in oesophageal cancer and the possible underlying mechanism are still unclear. The purpose of this study was to demonstrate the anticancer effect of CAR on oesophageal cancer in vivo and in vitro and to explore the underlying mechanism. Materials and Methods: MTT, crystal violet, and colony formation assays were used to detect oesophageal cancer cell proliferation. The effects of CAR on oesophageal cancer cell migration and invasion were detected by wound healing assay and Transwell assay. Hoechst 33258 staining and flow cytometry were used to detect cell apoptosis. Protein expression levels were detected by Western blot. A tumour xenograft model was established to further test the effect of CAR on the growth of oesophageal cancer in vivo. Results: The results showed that CAR inhibited the proliferation, migration, and invasion of oesophageal cancer cells in a concentration-dependent manner and induced apoptosis. Furthermore, the Western blot assay showed that CAR could suppress metastasis by inhibiting epithelial-mesenchymal transition (EMT) as indicated by downregulated expression of the mesenchymal markers N-cadherin and vimentin, the EMT transcription factor Snail, and matrix metalloproteinases (MMPs) and upregulated expression of the epithelial marker E-cadherin. CAR was associated with upregulation of the pro-apoptotic proteins Bax and Bad and downregulation of the anti-apoptotic protein Bcl-2 and triggered the mitochondrial apoptosis pathway, which in turn promoted caspase-3 activation and subsequent cleavage of PARP; however, the mitochondria-related apoptotic effects induced by CAR were blocked by caspase inhibitor Z-VAD-FMK pretreatment, which prevented programmed cell death triggered by CAR. In addition, CAR reduced the phosphorylation level of downstream effector molecules of phosphatidylinositol 3 kinase (PI3K) in a dose-dependent manner, and treatment with the PI3K agonist 740Y-P could partially reverse the anticancer effect of CAR, demonstrating that CAR played an antitumour role by inhibiting the PI3K/AKT signalling pathway in oesophageal cancer cells. Moreover, the EC9706 xenograft model further confirmed that CAR can significantly inhibit tumour growth in vivo. Conclusion: In summary, CAR exhibited a strong anticancer effect on human oesophageal cancer cells and promoted apoptosis by inhibiting the PI3K/AKT signalling pathway, suggesting that CAR can be used as new strategy for oesophageal cancer treatment.
Keywords: PI3K/AKT signalling pathway; antitumour; cardamonin; growth; oesophageal cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway.World J Gastrointest Oncol. 2022 Apr 15;14(4):872-886. doi: 10.4251/wjgo.v14.i4.872. World J Gastrointest Oncol. 2022. PMID: 35582102 Free PMC article.
-
Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway.Anticancer Drugs. 2019 Mar;30(3):241-250. doi: 10.1097/CAD.0000000000000709. Anticancer Drugs. 2019. PMID: 30640793
-
miR-15b, a diagnostic biomarker and therapeutic target, inhibits oesophageal cancer progression by regulating the PI3K/AKT signalling pathway.Exp Ther Med. 2020 Dec;20(6):222. doi: 10.3892/etm.2020.9352. Epub 2020 Oct 15. Exp Ther Med. 2020. PMID: 33363587 Free PMC article.
-
An overview of the potential anticancer properties of cardamonin.Explor Target Antitumor Ther. 2020;1(6):413-426. doi: 10.37349/etat.2020.00026. Epub 2020 Dec 28. Explor Target Antitumor Ther. 2020. PMID: 36046386 Free PMC article. Review.
-
Anti-cancer activity of guggulsterone by modulating apoptotic markers: a systematic review and meta-analysis.Front Pharmacol. 2023 May 2;14:1155163. doi: 10.3389/fphar.2023.1155163. eCollection 2023. Front Pharmacol. 2023. PMID: 37201024 Free PMC article.
Cited by
-
Cardamonin Promotes the Apoptosis and Chemotherapy Sensitivity to Gemcitabine of Pancreatic Cancer Through Modulating the FOXO3a-FOXM1 Axis.Dose Response. 2021 Dec 21;19(4):15593258211042163. doi: 10.1177/15593258211042163. eCollection 2021 Oct-Dec. Dose Response. 2021. PMID: 34987330 Free PMC article.
-
Natural Compounds Regulate Macrophage Polarization and Alleviate Inflammation Against ALI/ARDS.Biomolecules. 2025 Jan 29;15(2):192. doi: 10.3390/biom15020192. Biomolecules. 2025. PMID: 40001495 Free PMC article. Review.
-
A multi-targeting natural product, aiphanol, inhibits tumor growth and metastasis.Am J Cancer Res. 2022 Nov 15;12(11):4930-4953. eCollection 2022. Am J Cancer Res. 2022. PMID: 36504899 Free PMC article.
-
Mechanism of apoptosis in oral squamous cell carcinoma promoted by cardamonin through PI3K/AKT signaling pathway.Sci Rep. 2024 Sep 5;14(1):20802. doi: 10.1038/s41598-024-71817-1. Sci Rep. 2024. PMID: 39242879 Free PMC article.
-
Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence.Int J Mol Sci. 2023 Jun 19;24(12):10354. doi: 10.3390/ijms241210354. Int J Mol Sci. 2023. PMID: 37373500 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. - PubMed
-
- Abbasi BA, Iqbal J, Ahmad R, Bibi S, Mahmood T, Kanwal S. et al. Potential phytochemicals in the prevention and treatment of esophagus cancer: A green therapeutic approach. Pharmacol Rep. 2019;71:644–652. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

