Inner retinal injury in experimental glaucoma is prevented upon AAV mediated Shp2 silencing in a caveolin dependent manner
- PMID: 33995651
- PMCID: PMC8120201
- DOI: 10.7150/thno.55472
Inner retinal injury in experimental glaucoma is prevented upon AAV mediated Shp2 silencing in a caveolin dependent manner
Abstract
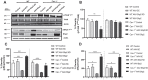
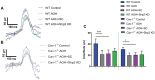
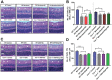
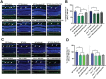
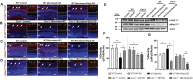
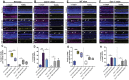
SH2 domain containing tyrosine phosphatase 2 (Shp2; PTPN11) regulates several intracellular pathways downstream of multiple growth factor receptors. Our studies implicate that Shp2 interacts with Caveolin-1 (Cav-1) protein in retinal ganglion cells (RGCs) and negatively regulates BDNF/TrkB signaling. This study aimed to investigate the mechanisms underlying the protective effects of shp2 silencing in the RGCs in glaucomatous conditions. Methods: Shp2 was silenced in the Cav-1 deficient mice and the age matched wildtype littermates using adeno-associated viral (AAV) constructs. Shp2 expression modulation was performed in an acute and a chronic mouse model of experimental glaucoma. AAV2 expressing Shp2 eGFP-shRNA under a strong synthetic CAG promoter was administered intravitreally in the animals' eyes. The contralateral eye received AAV-eGFP-scramble-shRNA as control. Animals with Shp2 downregulation were subjected to either microbead injections or acute ocular hypertension experimental paradigm. Changes in inner retinal function were evaluated by measuring positive scotopic threshold response (pSTR) while structural and biochemical alterations were evaluated through H&E staining, western blotting and immunohistochemical analysis of the retinal tissues. Results: A greater loss of pSTR amplitudes was observed in the WT mice compared to Cav-1-/- retinas in both the models. Silencing of Shp2 phosphatase imparted protection against inner retinal function loss in chronic glaucoma model in WT mice. The functional rescue also translated to structural preservation of ganglion cell layer in the chronic glaucoma condition in WT mice which was not evident in Cav-1-/- mice retinas. Conclusions: This study indicates that protective effects of Shp2 ablation under chronic experimental glaucoma conditions are dependent on Cav-1 in the retina, suggesting in vivo interactions between the two proteins.
Keywords: Caveolin; Glaucoma; Retinal Ganglion cells, Shp2 phosphatase; TrkB..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Casson RJ, Chidlow G, Wood JPMPM, Crowston JG, Goldberg I. Definition of glaucoma: Clinical and experimental concepts. Clin Exp Ophthalmol. 2012;40:341–9. - PubMed
-
- Flaxman SR, Bourne RRA, Resnikoff S. et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Heal. 2017;5:e1221–34. - PubMed
-
- Tham Y-CC, Li X, Wong TY, Quigley HA, Aung T, Cheng C-YY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. - PubMed
-
- Guymer C, Wood JP, Chidlow G, Casson RJ. Neuroprotection in glaucoma: recent advances and clinical translation. Clin Experiment Ophthalmol. 2019;47:88–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

