The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies
- PMID: 33995677
- PMCID: PMC8120225
- DOI: 10.7150/thno.55664
The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies
Abstract
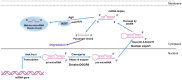
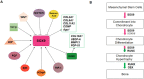
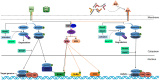
Mesenchymal stem cells (MSCs) have been identified in many adult tissues. MSCs can regenerate through cell division or differentiate into adipocytes, osteoblasts and chondrocytes. As a result, MSCs have become an important source of cells in tissue engineering and regenerative medicine for bone tissue and cartilage. Several epigenetic factors are believed to play a role in MSCs differentiation. Among these, microRNA (miRNA) regulation is involved in the fine modulation of gene expression during osteogenic/chondrogenic differentiation. It has been reported that miRNAs are involved in bone homeostasis by modulating osteoblast gene expression. In addition, countless evidence has demonstrated that miRNAs dysregulation is involved in the development of osteoporosis and bone fractures. The deregulation of miRNAs expression has also been associated with several malignancies including bone cancer. In this context, bone-associated circulating miRNAs may be useful biomarkers for determining the predisposition, onset and development of osteoporosis, as well as in clinical applications to improve the diagnosis, follow-up and treatment of cancer and metastases. Overall, this review will provide an overview of how miRNAs activities participate in osteogenic/chondrogenic differentiation, while addressing the role of miRNA regulatory effects on target genes. Finally, the role of miRNAs in pathologies and therapies will be presented.
Keywords: MSC differentiation; disease; epigenetics; microRNAs; tumour.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Manfrini M, Di Bona C, Canella A. et al. Mesenchymal stem cells from patients to assay bone graft substitutes. J Cell Physiol. 2013;228:1229–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

