Predictors of adverse drug reaction among adult HIV-infected patients on antiretroviral therapy in government hospitals of Kaffa Zone, Ethiopia; November 2018: a retrospective cohort
- PMID: 33995787
- PMCID: PMC8106778
- DOI: 10.11604/pamj.2021.38.181.19915
Predictors of adverse drug reaction among adult HIV-infected patients on antiretroviral therapy in government hospitals of Kaffa Zone, Ethiopia; November 2018: a retrospective cohort
Abstract
Introduction: incidence of adverse drug reactions (ADR) associated with antiretroviral therapy (ART) was higher in developing countries. In two teaching hospital in Ethiopia: Debremarkose 23% and Yirgalem 73.2% of study participants reported at least one ADR. Since there was limited information about ADR in the study area; we aimed to determine its incidence-rate and predictors.
Methods: we conducted retrospective cohort study using medical records of HIV-infected patients enrolled on ART between 2006 and 2017 in government hospitals of Ethiopia. ADR was defined as report of at least one unwanted response to ART. We run descriptive and cox regression analysis (CRA).
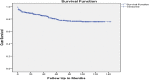
Results: incidence-rate of ADR was 4.1 per 100 person-years (py). Hazards of ADR among patients living at rural was almost two times than at urban; [Adjusted hazard ratio (AHR): 1.94(95% (CI): 1.18, 3.20)]. Stavudine (D4T)-Lamivudine (3TC)-Nevirapine (NVP) had about two times [AHR: 1.78(95%CI: 1.03, 3.08)], Zidovudine(AZT)-3TC-NVP had about two times [AHR: 2.34 (95%CI: 1.20, 4.57)], D4T-3TC-Efaviranze(EFV) had about three times [AHR: 2.86(95%CI: 1.38, 5.95)] and AZT-3TC-EFV had about two times [AHR: 2.16(95%CI: 1.21,3.90)] hazards of ADR than Tenofovir(TDF) based regimens. Being WHO clinical stage III had about two times hazard of ADR [AHR: 2.46 (95%CI: 1.22, 4.95)] and IV had about four times hazard of ADR [AHR: 4.32 (95%CI: 1.88, 9.93)] than stage I.
Conclusion: risk of ADR was higher among adult HIV-infected patients on ART living in rural, WHO clinical stage III and IV, and patients on AZT and D4T based regimen. AZT should not be given as an alternative treatment, increase access of TDF regimens.
Keywords: Antiretroviral therapy; Ethiopia; HIV/AIDS; adverse drug reaction.
Copyright: Ayanaw Ambachew Mitkie et al.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Fauci AS. 2017. HIV/AIDS USA: U.S. department of health and human survice, National institute of health.
-
- World Health Organization (WHO) THE GLOBAL HEALTH OBSERVATORY. Accessed on 22nd November 2017.
-
- AIDS info. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials