Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes
- PMID: 33995844
- PMCID: PMC8107972
- DOI: 10.4239/wjd.v12.i5.556
Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes
Abstract
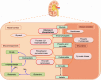
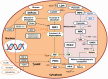
Renal gluconeogenesis is one of the major pathways for endogenous glucose production. Impairment in this process may contribute to hyperglycemia in cases with insulin resistance and diabetes. We reviewed pertinent studies to elucidate the role of renal gluconeogenesis regulation in insulin resistance and diabetes. A consensus on the suppressive effect of insulin on kidney gluconeogenesis has started to build up. Insulin-resistant models exhibit reduced insulin receptor (IR) expression and/or post-receptor signaling in their kidney tissue. Reduced IR expression or post-receptor signaling can cause impairment in insulin's action on kidneys, which may increase renal gluconeogenesis in the state of insulin resistance. It is now established that the kidney contributes up to 20% of all glucose production via gluconeogenesis in the post-absorptive phase. However, the rate of renal glucose release excessively increases in diabetes. The rise in renal glucose release in diabetes may contribute to fasting hyperglycemia and increased postprandial glucose levels. Enhanced glucose release by the kidneys and renal expression of the gluconeogenic-enzyme in diabetic rodents and humans further point towards the significance of renal gluconeogenesis. Overall, the available literature suggests that impairment in renal gluconeogenesis in an insulin-resistant state may contribute to hyperglycemia in type 2 diabetes.
Keywords: Diabetes; Gluconeogenic enzymes; Insulin; Insulin receptor signaling; Insulin-resistance; Renal gluconeogenesis.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflicts of interest.
Figures

References
-
- Björkman O, Felig P, Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980;29:610–616. - PubMed
-
- Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282:E428–E434. - PubMed
-
- Cersosimo E, Garlick P, Ferretti J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes. 2000;49:1186–1193. - PubMed
-
- Cersosimo E, Garlick P, Ferretti J. Renal glucose production during insulin-induced hypoglycemia in humans. Diabetes. 1999;48:261–266. - PubMed
-
- Meyer C, Dostou JM, Gerich JE. Role of the human kidney in glucose counterregulation. Diabetes. 1999;48:943–948. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

