DNA replication machinery: Insights from in vitro single-molecule approaches
- PMID: 33995902
- PMCID: PMC8085672
- DOI: 10.1016/j.csbj.2021.04.013
DNA replication machinery: Insights from in vitro single-molecule approaches
Abstract
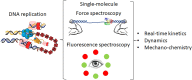
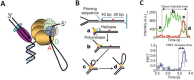
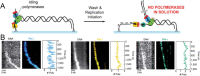
The replisome is the multiprotein molecular machinery that replicates DNA. The replisome components work in precise coordination to unwind the double helix of the DNA and replicate the two strands simultaneously. The study of DNA replication using in vitro single-molecule approaches provides a novel quantitative understanding of the dynamics and mechanical principles that govern the operation of the replisome and its components. 'Classical' ensemble-averaging methods cannot obtain this information. Here we describe the main findings obtained with in vitro single-molecule methods on the performance of individual replisome components and reconstituted prokaryotic and eukaryotic replisomes. The emerging picture from these studies is that of stochastic, versatile and highly dynamic replisome machinery in which transient protein-protein and protein-DNA associations are responsible for robust DNA replication.
Keywords: DNA replication; Fluorescence spectroscopy; Force spectroscopy; Replisome; Single-molecule.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Bessman M.J., Lehman I.R., Simms E.S., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. II. General properties of the reaction. The Journal of biological chemistry. 1958;233(1):171–177. Epub 1958/07/01. PubMed PMID: 13563463. - PubMed
-
- Lehman I.R., Bessman M.J., Simms E.S., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. The Journal of biological chemistry. 1958;233(1):163–170. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
