Immunogenicity evaluation of the HIV-1 Tat containing polyepitope DNA vaccine adjuvanted with CpG-ODNs in mice
- PMID: 33995949
- PMCID: PMC8087848
- DOI: 10.22038/ijbms.2021.52890.11923
Immunogenicity evaluation of the HIV-1 Tat containing polyepitope DNA vaccine adjuvanted with CpG-ODNs in mice
Abstract
Objectives: HIV-1 is still considered a serious threat to human health, and accessibility of a suitable and efficient vaccine is urgently needed to address the disease burden. DNA vaccines employ the cells of the vaccinated hosts for in situ production of the vaccines. This strategy is an alternative and effective approach for traditional vaccination against high-risk pathogens, e.g., HIV-1. On the other hand, polyepitope vaccines, containing several immunogenic and conserved epitopes from virus vital regulatory and structural proteins, could more efficiently induce cellular and humoral immune responses against different clades of the virus.
Materials and methods: Herein, we comparatively investigated the immunogenic potency of the HIV-1 polytope DNA vaccine containing CpG oligodeoxynucleotides (CpG-ODNs) in BALB/c mice. To this end, after verifying the expression of the recombinant sequence in the eukaryotic HEK 293 cell line, it was amplified and extracted in the prokaryotic host cells (E. coli DH5α)) and then formulated and administered intramuscularly (IM) to the experimental mice (on days 0, 14, and 28) with and without CpG-ODNs adjuvant.
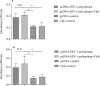
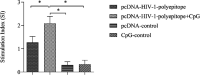
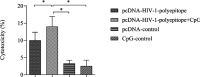
Results: Taken together, the results demonstrated that CpG-ODNs adjuvanted DNA vaccine could significantly elicit cellular and humoral immune responses in the immunized animals in comparison with the control ones (P<0.05).
Conclusion: Regarding the obtained results and also considering the advantages of polytopic and DNA vaccines, this approach might be considered a new regimen in HIV-1/AIDS vaccination.
Keywords: Cellular immunity; CpG oligodeoxynucleotides; DNA vaccine; HIV-1; Humoral immunity.
Figures


Similar articles
-
The effects of CpG-ODNs and Chitosan adjuvants on the elicitation of immune responses induced by the HIV-1-Tat-based candidate vaccines in mice.Pathog Dis. 2017 Mar 1;75(2). doi: 10.1093/femspd/ftx013. Pathog Dis. 2017. PMID: 28175274
-
Subcutaneous administration CpG-ODNs acts as a potent adjuvant for an HIV-1-tat-based vaccine candidate to elicit cellular immunity in BALB/c mice.Biotechnol Lett. 2018 Mar;40(3):527-533. doi: 10.1007/s10529-017-2497-9. Epub 2018 Jan 8. Biotechnol Lett. 2018. PMID: 29313255
-
Formulation of chitosan with the polyepitope HIV-1 protein candidate vaccine efficiently boosts cellular immune responses in mice.Pathog Dis. 2017 Nov 30;75(8). doi: 10.1093/femspd/ftx098. Pathog Dis. 2017. PMID: 28911033
-
Adjuvant activity of CpG oligodeoxynucleotides.Int Rev Immunol. 2006 May-Aug;25(3-4):135-54. doi: 10.1080/08830180600743057. Int Rev Immunol. 2006. PMID: 16818369 Review.
-
Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation.BioDrugs. 2007;21(6):387-401. doi: 10.2165/00063030-200721060-00006. BioDrugs. 2007. PMID: 18020622 Review.
References
-
- Girard MP, Plotkin SA. HIV vaccine development at the turn of the 21st century. Curr Opin HIV AIDS. 2012;7:4–9. - PubMed
-
- Habibzadeh N, Bolhassani A, Vahabpour R, Sadat SM. How can improve DNA vaccine modalities as a therapeutic approach against HIV infections? J AIDS Clin Res. 2015;6:1–8. - PubMed
-
- Lavanya J, Saxena S, Jais M, Dutta R. DNA Vaccines-A Review. Jeevanu Times. 2013;13:12–16.
-
- Liu M. DNA vaccines: A review. J Inter Med. 2003;253:402–410. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
