Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study
- PMID: 33995986
- PMCID: PMC8111519
- DOI: 10.1177/20406207211007058
Expert opinion on the UK standard of care for haemophilia patients with inhibitors: a modified Delphi consensus study
Abstract
Background and aims: Despite advances in haemophilia care, inhibitor development remains a significant complication. Although viable treatment options exist, there is some divergence of opinion in the appropriate standard approach to care and goals of treatment. The aim of this study was to assess consensus on United Kingdom (UK) standard of care for child and adult haemophilia patients with inhibitors.
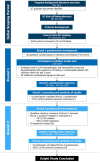
Methods: A modified Delphi study was conducted using a two-round online survey. A haemophilia expert steering committee and published literature informed the Round 1 questionnaire. Invited participants included haematologists, haemophilia nurses and physiotherapists who had treated at least one haemophilia patient with inhibitors in the past 5 years. Consensus for 6-point Likert scale questions was pre-defined as ⩾70% participants selecting 1-2 (disagreement) or 5-6 (agreement).
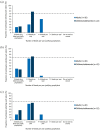
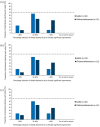
Results: In all, 46.7% and 35.9% questions achieved consensus in Rounds 1 (n = 41) and 2 (n = 34), respectively. Consensus was reached on the importance of improving quality of life (QoL) and reaching clinical goals such as bleed prevention, eradication of inhibitors and pain management. There was agreement on criteria constituting adequate/inadequate responses to immune tolerance induction (ITI) and the appropriate factor VIII dose to address suboptimal ITI response. Opinions varied on treatment aims for adults and children/adolescents, when to offer prophylaxis with bypassing agents and expectations of prophylaxis. Consensus was also lacking on appropriate treatment for mild/moderate patients with inhibitors.
Conclusion: UK healthcare professionals appear to be aligned on the clinical goals and role of ITI when managing haemophilia patients with inhibitors, although novel treatment developments may require reassessment of these goals. Lack of consensus on prophylaxis with bypassing agents and management of mild/moderate cases identifies a need for further research to establish more comprehensive, evidence-based treatment guidance, particularly for those patients who are unable/prefer not to receive non-factor therapies.
Keywords: Delphi panel; adult; children; consensus; haemophilia; inhibitors.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: KK: Research support from: Pfizer, Shire, Sobi; Speaker fees from Bayer, CSL Behring, Novo Nordisk, Roche, Shire and Sobi; EC: Research support from: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; Speaker’s fees: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; Education support: Boehringer Ingelheim, CSL Behring, Grifols, Roche, Shire and Sobi; TF: Education and quality improvement grants from: Sobi; Honoraria from: Bayer; AG: Employee of Costello Medical; FR: Employee of Roche Products Ltd; GT: Employee of Roche Products Ltd; PC: Research support from: Bayer, CSL Behring, Novo Nordisk, Pfizer and Sobi; Consulting fees from: Baxalta/Shire, Bayer, Biogen Idec, CSL Behring, Chugai, Freeline, Novo Nordisk, Pfizer, Roche and Sobi; Speaker’s bureau from: Baxalta/Shire, Biogen Idec, CSL Behring, Novo Nordisk, Pfizer, Roche and Sobi.
Figures

References
-
- Oldenburg J, Brackmann HH, Schwaab R. Risk factors for inhibitor development in hemophilia A. Haematologica 2000; 85: 7–13. - PubMed
-
- Mancuso ME, Mannucci PM, Rocino A, et al. Source and purity of factor VIII products as risk factors for inhibitor development in patients with hemophilia A. J Thromb Haemost 2012; 10: 781–790. - PubMed
-
- Ingerslev J. Hemophilia. Strategies for the treatment of inhibitor patients. Haematologica 2000; 85: 15–20. - PubMed
-
- Srivastava A, Brewer A, Mauser-Bunschoten E, et al. Guidelines for the management of hemophilia. Haemophilia 2013; 19: e1–e47. - PubMed
-
- Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Hematology Am Soc Hematol Educ Program 2014; 1: 364–371. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

