Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib
- PMID: 33995988
- PMCID: PMC8111531
- DOI: 10.1177/20406207211010875
Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib
Abstract
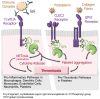
Background: Patients with immune thrombocytopenia (ITP) are at risk of bleeding and, paradoxically, thromboembolic events (TEEs), irrespective of thrombocytopenia. The risk of thrombosis is increased by advanced age, obesity, and prothrombotic comorbidities: cancer, hyperlipidemia, diabetes, hypertension, coronary artery disease, and chronic kidney disease, among others. Certain ITP treatments further increase the risk of TEE, especially splenectomy and thrombopoietin receptor agonists. Spleen tyrosine kinase (SYK) is a key signaling molecule common to thromboembolic and hemostatic (in addition to inflammatory) pathways. Fostamatinib is an orally administered SYK inhibitor approved in the USA and Europe for treatment of chronic ITP in adults.
Methods: The phase III and extension studies included heavily pretreated patients with long-standing ITP, many of whom had risk factors for thrombosis prior to initiating fostamatinib. This report describes long-term safety and efficacy of fostamatinib in 146 patients with up to 5 years of treatment, a total of 229 patient-years, and assesses the incidence of thromboembolic events (by standardized MedDRA query).
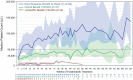
Results: Platelet counts ⩾50,000/µL were achieved in 54% of patients and the safety profile was as described in the phase III clinical studies with no new toxicities observed over the 5 years of follow-up. The only TEE occurred in one patient (0.7%, or 0.44/100 patient-years), who experienced a mild transient ischemic attack. This is a much lower rate than might be expected in ITP patients.
Conclusion: This report demonstrates durable efficacy and a very low incidence of TEE in patients receiving long-term treatment of ITP with the SYK inhibitor fostamatinib.
Clinicaltrialsgov identifiers: NCT02076399, NCT02076412, and NCT02077192.
Keywords: arterial thrombosis; disorders of platelet function; immune thrombocytopenic purpura; platelets; venous thrombosis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: NC: Amgen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. IA: Employed by Flatiron Health, Inc., an independent subsidiary of the Roche group; Bayer: Consultancy; Rigel: Consultancy; Amgen: Consultancy; Incyte: Consultancy, Research Funding; Novartis: Consultancy. PLRN, SPW, and MRT have received a research grant from Rigel. SPW is supported by the British Heart Foundation (03/003). PLRN received research grants from Novartis and Principia Biopharma; and honoraria from Bayer. EM, VM, and LKT are employed by Rigel Pharmaceuticals. JBB: Novartis: Consultancy; Argenx: Consultancy; UCB: Consultancy; CSLBehring: Consultancy; Shionogi: Consultancy; Regeneron: Consultancy; 3SBios: Consultancy; Dova: Consultancy; Principia: Consultancy; Rigel: Consultancy; Momenta: Consultancy; RallyBio: Consultancy; Amgen: Consultancy.
Figures

References
-
- Ekstrand C, Linder M, Baricault B, et al. Impact of risk factors on the occurrence of arterial thrombosis and venous thromboembolism in adults with primary immune thrombocytopenia - results from two nationwide cohorts. Thromb Res 2019; 178: 124–131. - PubMed
-
- Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol 2016; 91: 39–45. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

