A bifunctional iminophosphorane squaramide catalyzed enantioselective synthesis of hydroquinazolines via intramolecular aza-Michael reaction to α,β-unsaturated esters
- PMID: 33996002
- PMCID: PMC8098679
- DOI: 10.1039/d1sc00856k
A bifunctional iminophosphorane squaramide catalyzed enantioselective synthesis of hydroquinazolines via intramolecular aza-Michael reaction to α,β-unsaturated esters
Abstract
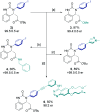
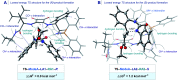
An efficient synthesis of enantioenriched hydroquinazoline cores via a novel bifunctional iminophosphorane squaramide catalyzed intramolecular aza-Michael reaction of urea-linked α,β-unsaturated esters is described. The methodology exhibits a high degree of functional group tolerance around the forming hydroquinazoline aryl core and wide structural variance on the nucleophilic N atom of the urea moiety. Excellent yields (up to 99%) and high enantioselectivities (up to 97 : 3 er) using both aromatic and less acidic aliphatic ureas were realized. The potential industrial applicability of the transformation was demonstrated in a 20 mmol scale-up experiment using an adjusted catalyst loading of 2 mol%. The origin of enantioselectivity and reactivity enhancement provided by the squaramide motif has been uncovered computationally using density functional theory (DFT) calculations, combined with the activation strain model (ASM) and energy decomposition analysis (EDA).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Bifunctional Iminophosphorane-Catalyzed Enantioselective Sulfa-Michael Addition to Unactivated α,β-Unsaturated Amides.J Am Chem Soc. 2022 Jan 19;144(2):1006-1015. doi: 10.1021/jacs.1c11898. Epub 2022 Jan 6. J Am Chem Soc. 2022. PMID: 34990142 Free PMC article.
-
Enantioselective bifunctional iminophosphorane catalyzed sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters.Chem Sci. 2017 Jan 1;8(1):606-610. doi: 10.1039/c6sc02878k. Epub 2016 Sep 14. Chem Sci. 2017. PMID: 28451207 Free PMC article.
-
Bifunctional cinchona alkaloid-squaramide-catalyzed highly enantioselective aza-Michael addition of indolines to α,β-unsaturated ketones.Tetrahedron Lett. 2013 Jul 3;54(27):3500-3502. doi: 10.1016/j.tetlet.2013.04.080. Tetrahedron Lett. 2013. PMID: 24014895 Free PMC article.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
Overlooked aza-S(IV) motifs: synthesis and transformations of sulfinamidines and sulfinimidate esters.Org Biomol Chem. 2023 Oct 4;21(38):7681-7690. doi: 10.1039/d3ob01382k. Org Biomol Chem. 2023. PMID: 37725053 Review.
Cited by
-
Catalytic Enantioselective Intramolecular Oxa-Michael Reaction to α,β-Unsaturated Esters and Amides.J Am Chem Soc. 2023 Jun 14;145(23):12771-12782. doi: 10.1021/jacs.3c03182. Epub 2023 May 30. J Am Chem Soc. 2023. PMID: 37253087 Free PMC article.
-
A Catalytic, Enantioselective Sulfamate Tethered Aza-Michael Cyclization.Org Lett. 2024 Dec 20;26(50):10708-10713. doi: 10.1021/acs.orglett.4c03558. Epub 2024 Dec 11. Org Lett. 2024. PMID: 39660506 Free PMC article.
-
Bifunctional Iminophosphorane-Catalyzed Enantioselective Sulfa-Michael Addition to Unactivated α,β-Unsaturated Amides.J Am Chem Soc. 2022 Jan 19;144(2):1006-1015. doi: 10.1021/jacs.1c11898. Epub 2022 Jan 6. J Am Chem Soc. 2022. PMID: 34990142 Free PMC article.
-
Bio-Based Polymer Developments from Tall Oil Fatty Acids by Exploiting Michael Addition.Polymers (Basel). 2022 Sep 28;14(19):4068. doi: 10.3390/polym14194068. Polymers (Basel). 2022. PMID: 36236017 Free PMC article.
-
Bifunctional Iminophosphorane-Catalyzed Enantioselective Nitroalkane Addition to Unactivated α,β-Unsaturated Esters.Angew Chem Int Ed Engl. 2023 May 15;62(21):e202303391. doi: 10.1002/anie.202303391. Epub 2023 Apr 13. Angew Chem Int Ed Engl. 2023. PMID: 36929179 Free PMC article.
References
-
- Shagufta W. Ahmad I. Med. Chem. Commun. 2017;8:871–885. - PMC - PubMed
- Tiwary B. K. Pradhan K. Nanda A. K. Chakraborty R. J. Chem. Biol. Ther. 2015;1:104.
- Patterson S. Alphey M. S. Jones D. C. Shanks E. J. Street I. P. Frearson J. A. Wyatt P. G. Gilbert I. H. Fairlamb A. H. J. Med. Chem. 2011;54:6514–6530. - PMC - PubMed
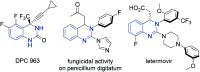
- Hemalatha K. Madhumitha G. Eur. J. Med. Chem. 2016;123:596–630. - PubMed
- Thanigaimalai P. Lee K.-C. Bang S.-C. Lee J.-H. Yun C.-Y. Roh E. Hwang B.-Y. Kim Y. Jung S.-H. Bioorg. Med. Chem. 2010;18:1555–1562. - PubMed
- Thanigaimalai P. Sharma V. K. Lee K.-C. Yun C.-Y. Kim Y. Jung S.-H. Bioorg. Med. Chem. Lett. 2010;20:4771–4773. - PubMed
- Hasegawa H. Muraoka M. Matsui K. Kojima A. Bioorg. Med. Chem. Lett. 2003;13:3471–3475. - PubMed
- Bernardelli P. Lorthiois E. Vergne F. Oliveira C. Mafroud A.-K. Proust E. Pham N. Ducrot P. Moreau F. Idrissi M. Tertre A. Bertin B. Coupe M. Chevalier E. Descours A. Berlioz-Seux F. Berna P. Li M. Bioorg. Med. Chem. Lett. 2004;14:4627–4631. - PubMed
- Gawad N. M. A. Georgey H. H. Youssef R. M. El-Sayed N. A. Eur. J. Med. Chem. 2010;45:6058–6067. - PubMed
- Byun J. S. Sohn J. M. Leem D. G. Park B. Nam J. H. Shin D. H. Shin J. S. Kim H. J. Lee K.-T. Lee J. Y. Bioorg. Med. Chem. Lett. 2016;26:1073–1079. - PubMed
- Patterson S. Alphey M. S. Jones D. C. Shanks E. J. Street I. P. Frearson J. A. Wyatt P. G. Gilbert I. H. Fairlamb A. H. J. Med. Chem. 2011;54:6514–6530. - PMC - PubMed
- Schlegel K.-A. S. Yang Z.-Q. Reger T. S. Shu Y. Cube R. Rittle K. E. Bondiskey P. Bock M. G. Hartman G. D. Tang C. Ballard J. Kuo Y. Prueksaritanont T. Nuss C. E. Doran S. M. Fox S. V. Garson S. L. Kraus R. L. Li Y. Uebele V. N. Renger J. J. Barrow J. C. Bioorg. Med. Chem. Lett. 2010;20:5147–5152. - PubMed
-
- King R. W. Klabe R. M. Reid C. D. Erickson-Viitanen S. K. Antimicrob. Agents Chemother. 2002;46:1640–1646. - PMC - PubMed
- Corbett J. W. Ko S. S. Rodgers J. D. Jeffrey S. Bacheler L. T. Klabe R. M. Diamond S. Lai C.-M. Rabel S. R. Saye J. A. Adams S. P. Trainor G. L. Anderson P. S. Erickson-Viitanen S. K. Antimicrob. Agents Chemother. 1999;43:2893–2897. - PMC - PubMed
- Corbett J. W. Ko S. S. Rodgers J. D. Gearhart L. A. Magnus N. A. Bacheler L. T. Diamond S. Jeffrey S. Klabe R. M. Cordova B. C. Garber S. Logue K. Trainor G. L. Anderson P. S. Erickson-Viitanen S. K. J. Med. Chem. 2000;43:2019–2030. - PubMed
-
- Li W. J. Li Q. Liu D. L. Ding M. W. J. Agric. Food Chem. 2013;61:1419–1426. - PubMed
-
- Pandey V. K. Kumar M. A. Trivedi N. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2008;47B:1910–1914.
- Seitz W. Geneste H. Backfisch G. Delzer J. Graef C. Hornberger W. Kling A. Subkowski T. Zimmermann N. Bioorg. Med. Chem. Lett. 2008;18:527–531. - PubMed
- Rhim H. Lee Y. S. Park S. J. Chung B. Y. Lee J. Y. Bioorg. Med. Chem. Lett. 2005;15:283–286. - PubMed
- Xie T. Xiao Y. Zhao S. Hu X.-Q. Xu P.-F. J. Org. Chem. 2016;81:10499–10505. - PubMed
- Willwacher J. Rakshit S. Glorius F. Org. Biomol. Chem. 2011;9:4736–4740. - PubMed
- Xin Z. Pei Z. Geldem T. V. Jirousek M. Tetrahedron Lett. 2000;41:1147–1150.
- Lee Y. S. Lee B. H. Park S. J. Kang S. B. Rhim H. Park J.-Y. Lee J.-H. Jeong S.-W. Lee J. Y. Bioorg. Med. Chem. Lett. 2004;14:3379–3384. - PubMed
- Ivachtchenko A. V. Kovalenko S. M. Drushlyak O. G. J. Comb. Chem. 2003;5:775–788. - PubMed
- Fukamachi S. Konishi H. Kobayashi K. Synthesis. 2010;10:1593–1598.
- Kobayashi K. Yokoi Y. Konishi H. Synthesis. 2011;10:1526–1528.
- Kaur B. Kaur R. ARKIVOC. 2007;15:315–323.
LinkOut - more resources
Full Text Sources
Other Literature Sources

