Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents
- PMID: 33996031
- PMCID: PMC8121267
- DOI: 10.1039/d0ra10610k
Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents
Abstract
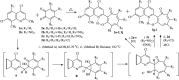
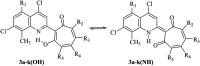
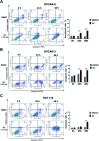
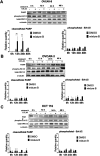
Tropolones are promising organic compounds that can have important biologic effects. We developed a series of new 2-quinolyl-1,3-tropolones derivatives that were prepared by the acid-catalyzed reaction of 4,7-dichloro-2-methylquinolines with 1,2-benzoquinones. 2-Quinolyl-1,3-tropolones have been synthesized and tested for their anti-proliferative activity against several human cancer cell lines. Two compounds (3d and mixture B of 3i-k) showed excellent activity against six cancer cell lines of different tissue of origin. The promising compounds 3d and mixture B of 3i-k also demonstrated induction of apoptotic cell death of ovarian cancer (OVCAR-3, OVCAR-8) and colon cancer (HCT 116) cell lines and affected ERK signaling. In summary, 2-quinolyl-1,3-tropolones are promising compounds for development of effective anticancer agents.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures

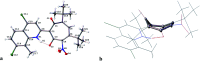



References
-
- Muñoz-Galván S. Felipe-Abrio B. García-Carrasco M. Domínguez-Piñol J. Suarez-Martinez E. Verdugo-Sivianes E. M. Espinosa-Sánchez A. Navas L. E. Otero-Albiol D. Marin J. J. Jiménez-García M. P. García-Heredia J. M. Quiroga A. G. Estevez-Garcia P. Carnero A. J. Exp. Clin. Cancer Res. 2019;38:234. doi: 10.1186/s13046-019-1245-5. - DOI - PMC - PubMed
-
- Guan L. Y. Lu Y. Discov. Med. 2018;26(144):219–229. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

