In-vivo mechanical characterization of coronary atherosclerotic plaques in living swine using intravascular laser speckle imaging
- PMID: 33996217
- PMCID: PMC8086462
- DOI: 10.1364/BOE.418939
In-vivo mechanical characterization of coronary atherosclerotic plaques in living swine using intravascular laser speckle imaging
Abstract
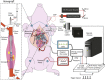
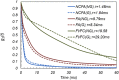
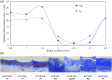
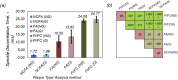
The ability to evaluate the viscoelastic properties of coronary arteries is crucial for identifying mechanically unstable atherosclerotic plaques. Here, we demonstrate for the first time in living swine, the capability of intravascular laser speckle imaging (ILSI) to measure an index of coronary plaque viscoelasticity, τ, using a human coronary to swine xenograft model. Cardiac motion effects are evaluated by comparing the EKG-non-gated , and EKG-gated among different plaque types. Results show that both and are significantly lower in necrotic-core plaques compared with stable lesions. Discrete-point pullback measurements demonstrate the capability of ILSI for rapid mechanical characterization of coronary segments under physiological conditions, in-vivo.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
ZH: Coalesenz Inc. (I, P) , SKN: Coalesenz Inc. (I, P, S).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
