Photoacoustic-guided surgery from head to toe [Invited]
- PMID: 33996218
- PMCID: PMC8086464
- DOI: 10.1364/BOE.417984
Photoacoustic-guided surgery from head to toe [Invited]
Abstract
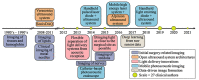
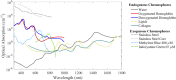
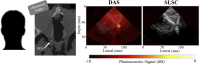
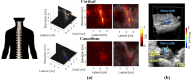
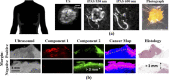
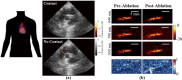
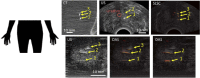
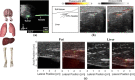
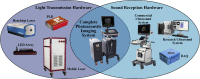
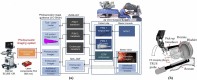
Photoacoustic imaging-the combination of optics and acoustics to visualize differences in optical absorption - has recently demonstrated strong viability as a promising method to provide critical guidance of multiple surgeries and procedures. Benefits include its potential to assist with tumor resection, identify hemorrhaged and ablated tissue, visualize metal implants (e.g., needle tips, tool tips, brachytherapy seeds), track catheter tips, and avoid accidental injury to critical subsurface anatomy (e.g., major vessels and nerves hidden by tissue during surgery). These benefits are significant because they reduce surgical error, associated surgery-related complications (e.g., cancer recurrence, paralysis, excessive bleeding), and accidental patient death in the operating room. This invited review covers multiple aspects of the use of photoacoustic imaging to guide both surgical and related non-surgical interventions. Applicable organ systems span structures within the head to contents of the toes, with an eye toward surgical and interventional translation for the benefit of patients and for use in operating rooms and interventional suites worldwide. We additionally include a critical discussion of complete systems and tools needed to maximize the success of surgical and interventional applications of photoacoustic-based technology, spanning light delivery, acoustic detection, and robotic methods. Multiple enabling hardware and software integration components are also discussed, concluding with a summary and future outlook based on the current state of technological developments, recent achievements, and possible new directions.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Manohar S., Vaartjes S. E., van Hespen J. C., Klaase J. M., van den Engh F. M., Steenbergen W., Van Leeuwen T. G., “Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics,” Opt. Express 15(19), 12277–12285 (2007). 10.1364/OE.15.012277 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
