Synchronously pumped Raman laser for simultaneous degenerate and nondegenerate two-photon microscopy
- PMID: 33996243
- PMCID: PMC8086478
- DOI: 10.1364/BOE.421647
Synchronously pumped Raman laser for simultaneous degenerate and nondegenerate two-photon microscopy
Abstract
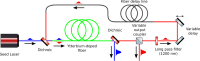
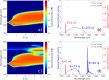
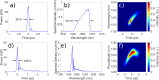
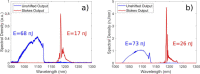
Two-photon fluorescence microscopy is a nonlinear imaging modality frequently used in deep-tissue imaging applications. A tunable-wavelength multicolor short-pulse source is usually required to excite fluorophores with a wide range of excitation wavelengths. This need is most typically met by solid-state lasers, which are bulky, expensive, and complicated systems. Here, we demonstrate a compact, robust fiber system that generates naturally synchronized femtosecond pulses at 1050 nm and 1200 nm by using a combination of gain-managed and Raman amplification. We image the brain of a mouse and view the blood vessels, neurons, and other cell-like structures using simultaneous degenerate and nondegenerate excitation.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures
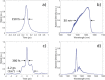
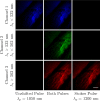
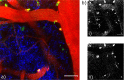
References
-
- Rakhymzhan A., Leben R., Zimmermann H., Günther R., Mex P., Reismann D., Ulbricht C., Acs A., Brandt A. U., Lindquist R. L., Winkler T. H., Hauser A. E., Niesner R. A., “Synergistic strategy for multicolor two-photon microscopy: application to the analysis of germinal center reactions in vivo,” Sci. Rep. 7(1), 7101 (2017). 10.1038/s41598-017-07165-0 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
