PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy
- PMID: 33996265
- PMCID: PMC8078506
- DOI: 10.1080/2162402X.2021.1912892
PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy
Abstract
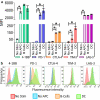
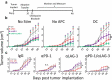
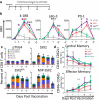
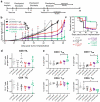
Concurrent blockade of different checkpoint receptors, notably PD-1 and CTLA-4, elicits greater anti-tumor activity for some tumor types, and the combination of different checkpoint receptor inhibitors is an active area of clinical research. We have previously demonstrated that anti-tumor vaccination, by activating CD8 + T cells, increases the expression of PD-1, CTLA-4, LAG-3 and other inhibitory receptors, and the anti-tumor efficacy of vaccination can be increased with checkpoint blockade. In the current study, we sought to determine whether anti-tumor vaccination might be further improved with combined checkpoint blockade. Using an OVA-expressing mouse tumor model, we found that CD8 + T cells activated in the presence of professional antigen presenting cells (APC) expressed multiple checkpoint receptors; however, T cells activated without APCs expressed LAG-3 alone, suggesting that LAG-3 might be a preferred target in combination with vaccination. Using three different murine tumor models, and peptide or DNA vaccines targeting three tumor antigens, we assessed the effects of vaccines with blockade of PD-1 and/or LAG-3 on tumor growth. We report that, in each model, the anti-tumor efficacy of vaccination was increased with PD-1 and/or LAG-3 blockade. However, combined PD-1 and LAG-3 blockade elicited the greatest anti-tumor effect when combined with vaccination in a MycCaP prostate cancer model in which PD-1 blockade alone with vaccination targeting a "self" tumor antigen had less efficacy. These results suggest anti-tumor vaccination might best be combined with concurrent blockade of both PD-1 and LAG-3, and potentially other checkpoint receptors whose expression is increased on CD8 + T cells following vaccine-mediated activation.
Keywords: APC; LAG-3; PD-1; tumor vaccine.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
Douglas G. McNeel has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc. which has licensed intellectual property related to this content. None of the other authors have relevant potential conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
