Impact of particle size and pH on protein corona formation of solid lipid nanoparticles: A proof-of-concept study
- PMID: 33996415
- PMCID: PMC8105779
- DOI: 10.1016/j.apsb.2020.10.023
Impact of particle size and pH on protein corona formation of solid lipid nanoparticles: A proof-of-concept study
Abstract
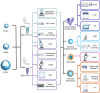
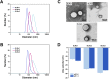
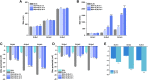
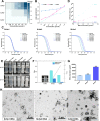
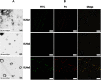
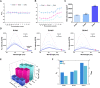
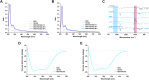
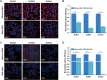
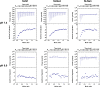
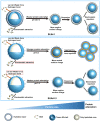
When nanoparticles were introduced into the biological media, the protein corona would be formed, which endowed the nanoparticles with new bio-identities. Thus, controlling protein corona formation is critical to in vivo therapeutic effect. Controlling the particle size is the most feasible method during design, and the influence of media pH which varies with disease condition is quite important. The impact of particle size and pH on bovine serum albumin (BSA) corona formation of solid lipid nanoparticles (SLNs) was studied here. The BSA corona formation of SLNs with increasing particle size (120-480 nm) in pH 6.0 and 7.4 was investigated. Multiple techniques were employed for visualization study, conformational structure study and mechanism study, etc. "BSA corona-caused aggregation" of SLN2‒3 was revealed in pH 6.0 while the dispersed state of SLNs was maintained in pH 7.4, which significantly affected the secondary structure of BSA and cell uptake of SLNs. The main interaction was driven by van der Waals force plus hydrogen bonding in pH 7.4, while by electrostatic attraction in pH 6.0, and size-dependent adsorption was confirmed. This study provides a systematic insight to the understanding of protein corona formation of SLNs.
Keywords: BSA corona-Caused aggregation; Cell uptake; Conformational structure; Medium pH; Nanoparticle-protein interaction; Protein corona; Size effect; Solid lipid nanoparticles.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Stefanick J.F., Omstead D.T., Kiziltepe T., Bilgicer B. Dual-receptor targeted strategy in nanoparticle design achieves tumor cell selectivity through cooperativity. Nanoscale. 2019;11:4414–4427. - PubMed
-
- Wang Y.B., Wu W.B., Liu J.J., Manghnani P.N., Hu F., Ma D., et al. Cancer-cell-activated photodynamic therapy assisted by Cu(II)-based metal-organic framework. ACS Nano. 2019;13:6879–6890. - PubMed
-
- Wan S.S., Cheng Q., Zeng X., Zhang X.Z. A Mn(III)-sealed metal-organic framework nanosystem for redox-unlocked tumor theranostics. ACS Nano. 2019;13:6561–6571. - PubMed
-
- Rezaei G., Mojtaba Daghighi S., Raoufi M., Esfandyari-Manesh M., Rahimifard M., Iranpur Mobarakeh V., et al. Synthetic and biological identities of polymeric nanoparticles influencing the cellular delivery: an immunological link. J Colloid Interface Sci. 2019;556:476–491. - PubMed
-
- Sousa F., Dhaliwal H.K., Gattacceca F., Sarmento B., Amiji M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J Control Release. 2019;309:37–47. - PubMed
LinkOut - more resources
Full Text Sources

