Molecular and epigenetic pathogenesis of germ cell tumors
- PMID: 33996469
- PMCID: PMC8099689
- DOI: 10.1016/j.ajur.2020.05.009
Molecular and epigenetic pathogenesis of germ cell tumors
Abstract
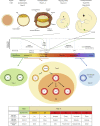
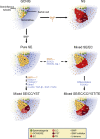
The development of germ cell tumors (GCTs) is a unique pathogenesis occurring at an early developmental stage during specification, migration or colonization of primordial germ cells (PGCs) in the genital ridge. Since driver mutations could not be identified so far, the involvement of the epigenetic machinery during the pathogenesis seems to play a crucial role. Currently, it is investigated whether epigenetic modifications occurring between the omnipotent two-cell stage and the pluripotent implanting PGCs might result in disturbances eventually leading to GCTs. Although progress in understanding epigenetic mechanisms during PGC development is ongoing, little is known about the complete picture of its involvement during GCT development and eventual classification into clinical subtypes. This review will shed light into the current knowledge of the complex epigenetic and molecular contribution during pathogenesis of GCTs by emphasizing on early developmental stages until arrival of late PGCs in the gonads. We questioned how misguided migrating and/or colonizing PGCs develop to either type I or type II GCTs. Additionally, we asked how pluripotency can be regulated during PGC development and which epigenetic changes contribute to GCT pathogenesis. We propose that SOX2 and SOX17 determine either embryonic stem cell-like (embryonal carcinoma) or PGC-like cell fate (seminoma). Finally, we suggest that factors secreted by the microenvironment, i.e. BMPs and BMP inhibiting molecules, dictate the fate decision of germ cell neoplasia in situ (into seminoma and embryonal carcinoma) and seminomas (into embryonal carcinoma or extraembryonic lineage), indicating an important role of the microenvironment on GCT plasticity.
Keywords: BMP signaling; Epigenetic reprogramming; Germ cell tumor; Microenvironment; Plasticity; Primordial germ cell; SOX17; SOX2.
© 2021 Editorial Office of Asian Journal of Urology. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Oosterhuis J.W., Looijenga L.H.J. Human germ cell tumours from a developmental perspective. Nat Rev Canc. 2019;19:522–537. - PubMed
-
- Fonseca A., Frazier A.L., Shaikh F. Germ cell tumors in adolescents and young adults. J Oncol Pract. 2019;15:433–441. - PubMed
-
- Baraban E.G., Cooper K. Pathogenesis of testicular germ cell neoplasia: a conceptual approach. Adv Anat Pathol. 2019;26:241–245. - PubMed
-
- Hernandez-Vargas H., Sincic N., Ouzounova M., Herceg Z. Epigenetic signatures in stem cells and cancer stem cells. Epigenomics. 2009;1:261–280. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

