NGFR Increases the Chemosensitivity of Colorectal Cancer Cells by Enhancing the Apoptotic and Autophagic Effects of 5-fluorouracil via the Activation of S100A9
- PMID: 33996571
- PMCID: PMC8120287
- DOI: 10.3389/fonc.2021.652081
NGFR Increases the Chemosensitivity of Colorectal Cancer Cells by Enhancing the Apoptotic and Autophagic Effects of 5-fluorouracil via the Activation of S100A9
Abstract
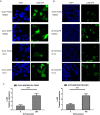
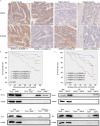
Colorectal cancer (CRC) is currently the third leading cause of cancer-related deaths worldwide, and 5-fluorouracil (5-FU)-based chemotherapies serve as important adjuvant therapies before and after surgery for CRC. However, the efficacy of CRC chemotherapy is limited by chemoresistance, and therefore the discovery of novel markers to indicate chemosensitivity is essential. Nerve growth factor receptor (NGFR), a cell surface receptor, is involved in cell death and survival. Our previous study indicated that NGFR acts as a tumor suppressor, and high expression is associated with better outcomes in patients receiving 5-FU-based adjuvant chemotherapy after surgery. The aim of this study was to investigate the effect of NGFR on the chemotherapeutic response in CRC. Chemosensitivity was investigated using DLD1 and HCT8 cells after NGFR transfection. Apoptosis was investigated by flow cytometry. Autophagy was assessed using GFP-LC3B transient transfection. Gene expression was measured using an mRNA microarray. Beclin-1 and Bcl-2 protein expressions were assessed by western blot. NGFR and S100 calcium-binding protein A9 (S100A9) expressions in CRC patients were investigated by immunohistochemistry. The results showed that the half maximal inhibitory concentration of NGFR-transfected cells was lower than that of controls in DLD1 and HCT8 cells after 5-FU treatment, and cell viability was lower than in empty-vector cells. Tumor sizes were also smaller than in empty-vector cells in vivo. The percentages of apoptotic and autophagic cells were higher in NGFR-transfected cells. NGFR elevated the expression of S100A9 after 5-FU treatment. The combination of Bcl-2 and Beclin-1 was significantly suppressed by overexpressed NGFR. Five-year overall and disease-free survival in NGFR+/S100A9+ patients was better than in NGFR-/S100A9- patients. This study's findings suggest that NGFR may serve as a marker predicting CRC patients' chemosensitivity.
Keywords: NGFR; S100A9; apoptosis; autophagy; chemosensitivity; colorectal cancer.
Copyright © 2021 Chen, Huang, Chen, Jiang, Feng, Liao and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures




Similar articles
-
Demethylzeylasteral Exerts Antitumor Effects via Disruptive Autophagic Flux and Apoptotic Cell Death in Human Colorectal Cancer Cells and Increases Cell Chemosensitivity to 5-Fluorouracil.Anticancer Agents Med Chem. 2022;22(5):851-863. doi: 10.2174/1871520621666210608104021. Anticancer Agents Med Chem. 2022. PMID: 34102996
-
Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer.BMC Cancer. 2018 Feb 20;18(1):207. doi: 10.1186/s12885-018-4123-1. BMC Cancer. 2018. PMID: 29463225 Free PMC article.
-
GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer.Biomed Pharmacother. 2018 May;101:945-952. doi: 10.1016/j.biopha.2018.03.028. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29635904
-
Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer.J Exp Clin Cancer Res. 2019 Jan 10;38(1):14. doi: 10.1186/s13046-018-0985-y. J Exp Clin Cancer Res. 2019. PMID: 30630498 Free PMC article.
-
MicroRNA-330 inhibited cell proliferation and enhanced chemosensitivity to 5-fluorouracil in colorectal cancer by directly targeting thymidylate synthase.Oncol Lett. 2017 May;13(5):3387-3394. doi: 10.3892/ol.2017.5895. Epub 2017 Mar 23. Oncol Lett. 2017. PMID: 28521444 Free PMC article.
Cited by
-
The CoREST complex is a therapeutic vulnerability in malignant peripheral nerve sheath tumors.Sci Rep. 2025 Mar 24;15(1):10128. doi: 10.1038/s41598-025-94517-w. Sci Rep. 2025. PMID: 40128216 Free PMC article.
-
Natural Alternatives in the Treatment of Colorectal Cancer: A Mechanisms Perspective.Biomolecules. 2025 Feb 24;15(3):326. doi: 10.3390/biom15030326. Biomolecules. 2025. PMID: 40149862 Free PMC article. Review.
-
Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer.Pharmaceuticals (Basel). 2025 Jul 17;18(7):1053. doi: 10.3390/ph18071053. Pharmaceuticals (Basel). 2025. PMID: 40732340 Free PMC article.
-
Identification and validation of novel biomarkers affecting bladder cancer immunotherapy via machine learning and its association with M2 macrophages.Front Immunol. 2022 Nov 9;13:1051063. doi: 10.3389/fimmu.2022.1051063. eCollection 2022. Front Immunol. 2022. PMID: 36439109 Free PMC article.
-
HSPA8 regulates anti-bacterial autophagy through liquid-liquid phase separation.Autophagy. 2023 Oct;19(10):2702-2718. doi: 10.1080/15548627.2023.2223468. Epub 2023 Jun 13. Autophagy. 2023. PMID: 37312409 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

