Repositioning of Antiparasitic Drugs for Tumor Treatment
- PMID: 33996598
- PMCID: PMC8117216
- DOI: 10.3389/fonc.2021.670804
Repositioning of Antiparasitic Drugs for Tumor Treatment
Abstract
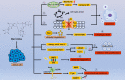
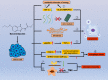
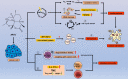
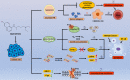
Drug repositioning is a strategy for identifying new antitumor drugs; this strategy allows existing and approved clinical drugs to be innovatively repurposed to treat tumors. Based on the similarities between parasitic diseases and cancer, recent studies aimed to investigate the efficacy of existing antiparasitic drugs in cancer. In this review, we selected two antihelminthic drugs (macrolides and benzimidazoles) and two antiprotozoal drugs (artemisinin and its derivatives, and quinolines) and summarized the research progresses made to date on the role of these drugs in cancer. Overall, these drugs regulate tumor growth via multiple targets, pathways, and modes of action. These antiparasitic drugs are good candidates for comprehensive, in-depth analyses of tumor occurrence and development. In-depth studies may improve the current tumor diagnoses and treatment regimens. However, for clinical application, current investigations are still insufficient, warranting more comprehensive analyses.
Keywords: antiparasitic drugs; artemisinin; autophagy; benzimidazoles; drug repositioning; ferroptosis; macrolides; quinolines.
Copyright © 2021 Li, Zheng, Liu, Lu, Zhou, Zhang, Zheng and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

