The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions-Evidence for Estrogen as an Immunomodulator
- PMID: 33996630
- PMCID: PMC8120286
- DOI: 10.3389/fcimb.2021.649815
The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions-Evidence for Estrogen as an Immunomodulator
Abstract
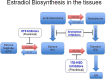
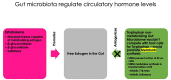
Globally, human papilloma virus (HPV) infection is a common sexually transmitted disease. However, most of the HPV infections eventually resolve aided by the body's efficient cell-mediated immune responses. In the vast majority of the small group of patients who develop overt disease too, it is the immune response that culminates in regression of lesions. It is therefore a rarity that persistent infection by high-risk genotypes of HPV compounded by other risk factors progresses through precancer (various grades of cervical intraepithelial neoplasia-CIN) to cervical cancer (CxCa). Hence, although CxCa is a rare culmination of HPV infection, the latter is nevertheless causally linked to >90% of cancer. The three 'Es' of cancer immunoediting viz. elimination, equilibrium, and escape come into vogue during the gradual evolution of CIN 1 to CxCa. Both cell-intrinsic and extrinsic mechanisms operate to eliminate virally infected cells: cell-extrinsic players are anti-tumor/antiviral effectors like Th1 subset of CD4+ T cells, CD8+ cytotoxic T cells, Natural Killer cells, etc. and pro-tumorigenic/immunosuppressive cells like regulatory T cells (Tregs), Myeloid-Derived Suppressor Cells (MDSCs), type 2 macrophages, etc. And accordingly, when immunosuppressive cells overpower the effectors e.g., in high-grade lesions like CIN 2 or 3, the scale is tilted towards immune escape and the disease progresses to cancer. Estradiol has long been considered as a co-factor in cervical carcinogenesis. In addition to the gonads, the Peyer's patches in the gut synthesize estradiol. Over and above local production of the hormone in the tissues, estradiol metabolism by the gut microbiome: estrobolome versus tryptophan non-metabolizing microbiome, regulates free estradiol levels in the intestine and extraintestinal mucosal sites. Elevated tissue levels of the hormone serve more than one purpose: besides a direct growth-promoting action on cervical epithelial cells, estradiol acting genomically via Estrogen Receptor-α also boosts the function of the stromal and infiltrating immunosuppressive cells viz. Tregs, MDSCs, and carcinoma-associated fibroblasts. Hence as a corollary, therapeutic repurposing of Selective Estrogen Receptor Disruptors or aromatase inhibitors could be useful for modulating immune function in cervical precancer/cancer. The immunomodulatory role of estradiol in HPV-mediated cervical lesions is reviewed.
Keywords: carcinoma-associated fibroblasts; cervical cancer microenvironment; cervical intraepithelial neoplasia (CIN); estrogen; human papilloma virus; myeloid-derived suppressor cells; regulatory T cells; selective estrogen receptor disruptors.
Copyright © 2021 R. S.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):E3255-64. doi: 10.1073/pnas.1509322112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056290 Free PMC article.
-
Cell intrinsic & extrinsic factors in cervical carcinogenesis.Indian J Med Res. 2009 Sep;130(3):286-95. Indian J Med Res. 2009. PMID: 19901438 Review.
-
Prevalence of Specific Types of Human Papiloma Virus in Cervical Intraepithelial Lesions and Cervical Cancer in Macedonian Women.Med Arch. 2018 Feb;72(1):26-30. doi: 10.5455/medarh.2018.72.26-30. Med Arch. 2018. PMID: 29416214 Free PMC article.
-
Infiltrating CD4 and CD8 lymphocytes in HPV infected uterine cervical milieu.Cancer Manag Res. 2019 Aug 14;11:7647-7655. doi: 10.2147/CMAR.S217264. eCollection 2019. Cancer Manag Res. 2019. PMID: 31616181 Free PMC article.
-
Precancer of the human cervix.Cancer Surv. 1998;32:201-29. Cancer Surv. 1998. PMID: 10489629 Review.
Cited by
-
Epigenetic and Genetic Keys to Fight HPV-Related Cancers.Cancers (Basel). 2023 Nov 25;15(23):5583. doi: 10.3390/cancers15235583. Cancers (Basel). 2023. PMID: 38067286 Free PMC article. Review.
-
Human Papillomavirus in Breast Carcinogenesis: A Passenger, a Cofactor, or a Causal Agent?Biology (Basel). 2021 Aug 20;10(8):804. doi: 10.3390/biology10080804. Biology (Basel). 2021. PMID: 34440036 Free PMC article. Review.
-
Peripheral blood immune cell parameters in patients with high-grade squamous intraepithelial lesion (HSIL) and cervical cancer and their clinical value: a retrospective study.PeerJ. 2024 Jun 3;12:e17499. doi: 10.7717/peerj.17499. eCollection 2024. PeerJ. 2024. PMID: 38846752 Free PMC article.
-
High Rates of High-risk HPV Anal Infection and Abnormal Cytology in a Cohort of Transgender People Assigned Male at Birth.Open Forum Infect Dis. 2024 Nov 12;11(12):ofae662. doi: 10.1093/ofid/ofae662. eCollection 2024 Dec. Open Forum Infect Dis. 2024. PMID: 39679352 Free PMC article.
-
Review Article: Immune Landscape and Immunotherapy Options in Cervical Carcinoma.Cancers (Basel). 2022 Sep 14;14(18):4458. doi: 10.3390/cancers14184458. Cancers (Basel). 2022. PMID: 36139618 Free PMC article. Review.
References
-
- Adurthi S., Krishna S., Mukherjee G., Bafna U. D., Devi U., Jayshree R. S. (2008). Regulatory T Cells in a Spectrum of HPV-Induced Cervical Lesions: Cervicitis, Cervical Intraepithelial Neoplasia and Squamous Cell Carcinoma. Am. J. Reprod. Immunol. 60, 55–65. 10.1111/j.1600-0897.2008.00590.x - DOI - PubMed
-
- Adurthi S., Mukherjee G., Krishnamurthy H., Sudhir K., Bafna U. D., Umadevi K., et al. . (2012). Functional Tumor Infiltrating TH1 and TH2 Effectors in Large Early-Stage Cervical Cancer are Suppressed by Regulatory T Cells. Int. J. Gynecol. Cancer. 22, 1130–1137. 10.1097/IGC.0b013e318262aa53 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

