Structural Insights of Shigella Translocator IpaB and Its Chaperone IpgC in Solution
- PMID: 33996640
- PMCID: PMC8117225
- DOI: 10.3389/fcimb.2021.673122
Structural Insights of Shigella Translocator IpaB and Its Chaperone IpgC in Solution
Abstract
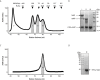
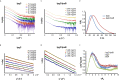
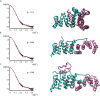
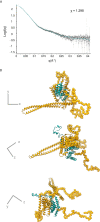
Bacterial Type III Secretion Systems (T3SSs) are specialized multicomponent nanomachines that mediate the transport of proteins either to extracellular locations or deliver Type III Secretion effectors directly into eukaryotic host cell cytoplasm. Shigella, the causing agent of bacillary dysentery or shigellosis, bears a set of T3SS proteins termed translocators that form a pore in the host cell membrane. IpaB, the major translocator of the system, is a key factor in promoting Shigella pathogenicity. Prior to secretion, IpaB is maintained inside the bacterial cytoplasm in a secretion competent folding state thanks to its cognate chaperone IpgC. IpgC couples T3SS activation to transcription of effector genes through its binding to MxiE, probably after the delivery of IpaB to the secretion export gate. Small Angle X-ray Scattering experiments and modeling reveal that IpgC is found in different oligomeric states in solution, as it forms a stable heterodimer with full-length IpaB in contrast to an aggregation-prone homodimer in the absence of the translocator. These results support a stoichiometry of interaction 1:1 in the IpgC/IpaB complex and the multi-functional nature of IpgC under different T3SS states.
Keywords: IpaB translocator; IpgC chaperone; Shigella flexneri; small angle x-ray scattering; type III secretion (T3S); type III translocator.
Copyright © 2021 Ferrari, Charova, Sansonetti, Mylonas and Gazi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Barta M. L., Tachiyama S., Muthuramalingam M., Arizmendi O., Villanueva C. E., Ramyar K. X., et al. (2018). Using Disruptive Insertional Mutagenesis to Identify the in Situ Structure-Function Landscape of the Shigella Translocator Protein Ipab. Protein Sci. 27, 1392–1406. 10.1002/pro.3428 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

