Structure Activity Relationship of N-Substituted Phenyldihydropyrazolones Against Trypanosoma cruzi Amastigotes
- PMID: 33996737
- PMCID: PMC8120161
- DOI: 10.3389/fchem.2021.608438
Structure Activity Relationship of N-Substituted Phenyldihydropyrazolones Against Trypanosoma cruzi Amastigotes
Abstract
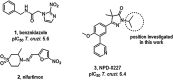
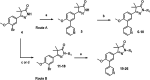
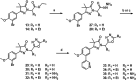
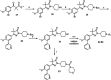
Current drugs for Chagas disease have long treatment regimens with occurrence of adverse drug effects leading to poor treatment compliance. Novel and efficacious medications are therefore highly needed. We previously reported on the discovery of NPD-0227 (2-isopropyl-5-(4-methoxy-3-(pyridin-3-yl)phenyl)-4,4-dimethyl-2,4-dihydro-3H-pyrazol-3-one) as a potent in vitro inhibitor of Trypanosoma cruzi (pIC50 = 6.4) with 100-fold selectivity over human MRC-5 cells. The present work describes a SAR study on the exploration of substituents on the phenylpyrazolone nitrogen. Modifications were either done directly onto this pyrazolone nitrogen or alternatively by introducing a piperidine linker. Attention was pointed toward the selection of substituents with a cLogP preferably below NPD-0227s cLogP of 3.5. Generally the more apolar compounds showed better activities then molecules with cLogPs <2.0. Several new compounds were identified with potencies that are in the same range as NPD-0227 (pIC50 = 6.4) and promising selectivities. While the potency could not be improved, valuable SAR was obtained. Furthermore the introduction of a piperidine linker offers new opportunities for derivatization as valuable novel starting points for future T. cruzi drug discovery.
Keywords: chagas disease; phenotypic optmization; phenylpyrazolones; structure activity relationship; trypanosama cruzi.
Copyright © 2021 Sijm, Maes, de Esch, Caljon, Sterk and Leurs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

