Application of Amino Acids in the Structural Modification of Natural Products: A Review
- PMID: 33996749
- PMCID: PMC8118163
- DOI: 10.3389/fchem.2021.650569
Application of Amino Acids in the Structural Modification of Natural Products: A Review
Abstract
Natural products and their derivatives are important sources for drug discovery; however, they usually have poor solubility and low activity and require structural modification. Amino acids are highly soluble in water and have a wide range of activities. The introduction of amino acids into natural products is expected to improve the performance of these products and minimize their adverse effects. Therefore, this review summarizes the application of amino acids in the structural modification of natural products and provides a theoretical basis for the structural modification of natural products in the future. The articles were divided into six types based on the backbone structures of the natural products, and the related applications of amino acids in the structural modification of natural products were discussed in detail.
Keywords: amino acids; derivatives; natural products; research progress; structure modification.
Copyright © 2021 Xu, Deng, Li and Quan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

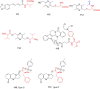
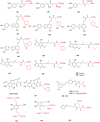
Similar articles
-
Application of cinnamic acid in the structural modification of natural products: A review.Phytochemistry. 2023 Feb;206:113532. doi: 10.1016/j.phytochem.2022.113532. Epub 2022 Dec 5. Phytochemistry. 2023. PMID: 36470328 Review.
-
The structural modification of natural products for novel drug discovery.Expert Opin Drug Discov. 2017 Feb;12(2):121-140. doi: 10.1080/17460441.2016.1272757. Epub 2016 Dec 22. Expert Opin Drug Discov. 2017. PMID: 28006993 Review.
-
Recent progress on betulinic acid and its derivatives as antitumor agents: a mini review.Chin J Nat Med. 2021 Sep;19(9):641-647. doi: 10.1016/S1875-5364(21)60097-3. Chin J Nat Med. 2021. PMID: 34561074 Review.
-
The RaPID Platform for the Discovery of Pseudo-Natural Macrocyclic Peptides.Acc Chem Res. 2021 Sep 21;54(18):3604-3617. doi: 10.1021/acs.accounts.1c00391. Epub 2021 Sep 10. Acc Chem Res. 2021. PMID: 34505781
-
Progress in Research of Chitosan Chemical Modification Technologies and Their Applications.Mar Drugs. 2022 Aug 21;20(8):536. doi: 10.3390/md20080536. Mar Drugs. 2022. PMID: 36005539 Free PMC article. Review.
Cited by
-
Development of trisubstituted thiophene-3-arboxamide selenide derivatives as novel EGFR kinase inhibitors with cytotoxic activity.RSC Med Chem. 2023 Oct 7;14(12):2677-2698. doi: 10.1039/d3md00403a. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107169 Free PMC article.
-
Modular Synthesis of Unnatural Peptides via Rh(III)-Catalyzed Diastereoselective Three-Component Carboamidation Reaction.J Am Chem Soc. 2023 Jan 18;145(2):1129-1135. doi: 10.1021/jacs.2c10793. Epub 2022 Dec 28. J Am Chem Soc. 2023. PMID: 36576945 Free PMC article.
-
Evaluation of Antitumor Activity of Xanthones Conjugated with Amino Acids.Int J Mol Sci. 2024 Feb 9;25(4):2121. doi: 10.3390/ijms25042121. Int J Mol Sci. 2024. PMID: 38396802 Free PMC article.
-
Natural products in osteoarthritis treatment: bridging basic research to clinical applications.Chin Med. 2024 Feb 15;19(1):25. doi: 10.1186/s13020-024-00899-w. Chin Med. 2024. PMID: 38360724 Free PMC article. Review.
-
QSPR analysis of amino acids for the family of Gourava indices.PLoS One. 2025 Apr 29;20(4):e0319029. doi: 10.1371/journal.pone.0319029. eCollection 2025. PLoS One. 2025. PMID: 40300030 Free PMC article.
References
-
- Baloch S. K., Ma L., Wang X. L., Shi J., Zhu Y., Wu F. Y., et al. . (2015). Design, synthesis and mechanism of novel shikonin derivatives as potent anticancer agents. Rsc. Adv. 5, 31759–31767. 10.1039/C5RA01872B - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

