Isocyanides: Promising Functionalities in Bioorthogonal Labeling of Biomolecules
- PMID: 33996762
- PMCID: PMC8117350
- DOI: 10.3389/fchem.2021.670751
Isocyanides: Promising Functionalities in Bioorthogonal Labeling of Biomolecules
Abstract
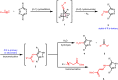
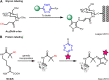
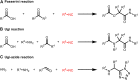
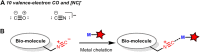
Isocyanides have drawn increasing attention in biological applications due to their attractive properties and unique reactivities, which can undergo various reactions, such as multicomponent reactions, α-addition reactions, [4 + 1] cycloaddition reactions, and the reaction scope keeps expanding. In addition to acting as reactants for the preparation of structurally interesting and diverse N-heterocycles or peptidomimetics, this type of functionality may be a good choice in the labeling and modulation of biomolecules due to the high biocompatibility and small size to minimize modifications on the parent molecule. It has been demonstrated that isocyanides can participate in biomolecule labeling through three strategies, including the two-component bioorthogonal reaction, multicomponent reaction, and metal chelation. Among them, the isocyanide-tetrazine reaction has been better studied recently, augmenting the potency of isocyanide as a bioorthogonal handle. This review will focus on the recent progress in isocyanide chemistry for labeling of biomolecules. Meanwhile, methods to introduce isocyano groups into biomacromolecules are also described to facilitate wider applications of this unique functionality.
Keywords: biomolecule labeling; bioorthogonal chemistry; cleavage; isocyanide; ligation.
Copyright © 2021 Zhu, Liao and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Addition of Isocyanide-Containing Amino Acids to the Genetic Code for Protein Labeling and Activation.ACS Chem Biol. 2019 Dec 20;14(12):2793-2799. doi: 10.1021/acschembio.9b00678. Epub 2019 Nov 14. ACS Chem Biol. 2019. PMID: 31682403 Free PMC article.
-
From mechanism to mouse: a tale of two bioorthogonal reactions.Acc Chem Res. 2011 Sep 20;44(9):666-76. doi: 10.1021/ar200148z. Epub 2011 Aug 15. Acc Chem Res. 2011. PMID: 21838330 Free PMC article.
-
Bioorthogonal chemistry: strategies and recent developments.Chem Commun (Camb). 2013 Dec 7;49(94):11007-22. doi: 10.1039/c3cc44272a. Chem Commun (Camb). 2013. PMID: 24145483 Free PMC article. Review.
-
Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics.Beilstein J Org Chem. 2014 Mar 4;10:544-98. doi: 10.3762/bjoc.10.50. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 24605172 Free PMC article. Review.
-
Enantiopure β-isocyano-boronic esters: synthesis and exploitation in isocyanide-based multicomponent reactions.Mol Divers. 2023 Oct;27(5):2161-2168. doi: 10.1007/s11030-022-10549-8. Epub 2022 Oct 19. Mol Divers. 2023. PMID: 36258147 Free PMC article.
Cited by
-
The Evolution of Fluorescein into A Potential Theranostic Tool.Chemistry. 2025 Jun 17;31(34):e202501513. doi: 10.1002/chem.202501513. Epub 2025 May 13. Chemistry. 2025. PMID: 40317604 Free PMC article.
-
Biosynthesis of isonitrile lipopeptides.Curr Opin Chem Biol. 2024 Aug;81:102470. doi: 10.1016/j.cbpa.2024.102470. Epub 2024 May 23. Curr Opin Chem Biol. 2024. PMID: 38788523 Free PMC article. Review.
-
Crystal Clear: Decoding Isocyanide Intermolecular Interactions through Crystallography.J Org Chem. 2024 Jan 19;89(2):957-974. doi: 10.1021/acs.joc.3c02038. Epub 2024 Jan 4. J Org Chem. 2024. PMID: 38175810 Free PMC article.
References
-
- Alday J., Mazzeo A., Suarez S. (2020). Selective Detection of Gasotransmitters Using Fluorescent Probes Based on Transition Metal Complexes. Inorg. Chim. Acta 510, 119696. 10.1016/j.ica.2020.119696 - DOI
-
- Bode M. L., Gravestock D., Rousseau A. L. (2016). Synthesis, Reactions and Uses of Isocyanides in Organic Synthesis. An Update. Org. Preparations Procedures Int. 48, 89–221. 10.1080/00304948.2016.1138072 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

