Superresolution Microscopy Reveals Distinct Phosphoinositide Subdomains Within the Cilia Transition Zone
- PMID: 33996795
- PMCID: PMC8120242
- DOI: 10.3389/fcell.2021.634649
Superresolution Microscopy Reveals Distinct Phosphoinositide Subdomains Within the Cilia Transition Zone
Abstract
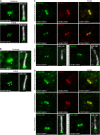
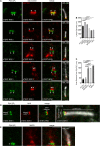
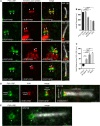
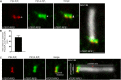
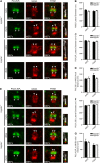
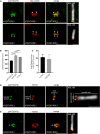
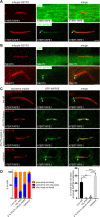
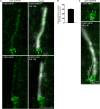
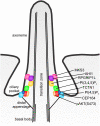
Primary cilia are evolutionary conserved microtubule-based organelles that protrude from the surface of most mammalian cells. Phosphoinositides (PI) are membrane-associated signaling lipids that regulate numerous cellular events via the recruitment of lipid-binding effectors. The temporal and spatial membrane distribution of phosphoinositides is regulated by phosphoinositide kinases and phosphatases. Recently phosphoinositide signaling and turnover has been observed at primary cilia. However, the precise localization of the phosphoinositides to specific ciliary subdomains remains undefined. Here we use superresolution microscopy (2D stimulated emission depletion microscopy) to map phosphoinositide distribution at the cilia transition zone. PI(3,4,5)P3 and PI(4,5)P2 localized to distinct subregions of the transition zone in a ring-shape at the inner transition zone membrane. Interestingly, the PI(3,4,5)P3 subdomain was more distal within the transition zone relative to PtdIns(4,5)P2. The phosphoinositide effector kinase pAKT(S473) localized in close proximity to these phosphoinositides. The inositol polyphosphate 5-phosphatase, INPP5E, degrades transition zone phosphoinositides, however, studies of fixed cells have reported recombinant INPP5E localizes to the ciliary axoneme, distant from its substrates. Notably, here using live cell imaging and optimized fixation/permeabilization protocols INPP5E was found concentrated at the cilia base, in a distribution characteristic of the transition zone in a ring-shaped domain of similar dimensions to the phosphoinositides. Collectively, this superresolution map places the phosphoinositides in situ with the transition zone proteins and reveals that INPP5E also likely localizes to a subdomain of the transition zone membrane, where it is optimally situated to control local phosphoinositide metabolism.
Keywords: INPP5E; phosphoinositides; primary cilia; superresolution microscopy; transition zone.
Copyright © 2021 Conduit, Davies, Fulcher, Oorschot and Mitchell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
INPP5E Regulates the Distribution of Phospholipids on Cilia in RPE1 Cells.J Clin Lab Anal. 2024 Apr;38(7):e25031. doi: 10.1002/jcla.25031. Epub 2024 Mar 21. J Clin Lab Anal. 2024. PMID: 38514901 Free PMC article.
-
A compartmentalized phosphoinositide signaling axis at cilia is regulated by INPP5E to maintain cilia and promote Sonic Hedgehog medulloblastoma.Oncogene. 2017 Oct 26;36(43):5969-5984. doi: 10.1038/onc.2017.208. Epub 2017 Jun 26. Oncogene. 2017. PMID: 28650469
-
Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling.Dev Cell. 2015 Aug 24;34(4):400-409. doi: 10.1016/j.devcel.2015.08.001. Dev Cell. 2015. PMID: 26305592 Free PMC article.
-
INPP5E and Coordination of Signaling Networks in Cilia.Front Mol Biosci. 2022 Apr 6;9:885592. doi: 10.3389/fmolb.2022.885592. eCollection 2022. Front Mol Biosci. 2022. PMID: 35463949 Free PMC article. Review.
-
Inositol polyphosphate 5-phosphatases; new players in the regulation of cilia and ciliopathies.FEBS Lett. 2012 Aug 31;586(18):2846-57. doi: 10.1016/j.febslet.2012.07.037. Epub 2012 Jul 22. FEBS Lett. 2012. PMID: 22828281 Review.
Cited by
-
Cholesterol and Phosphoinositides in Cilia Biology.Adv Exp Med Biol. 2023;1422:121-142. doi: 10.1007/978-3-031-21547-6_4. Adv Exp Med Biol. 2023. PMID: 36988879 Review.
-
Single-molecule imaging in the primary cilium.Methods Cell Biol. 2023;176:59-83. doi: 10.1016/bs.mcb.2023.01.003. Epub 2023 Feb 24. Methods Cell Biol. 2023. PMID: 37164543 Free PMC article.
-
Multiple ciliary localization signals control INPP5E ciliary targeting.Elife. 2022 Sep 5;11:e78383. doi: 10.7554/eLife.78383. Elife. 2022. PMID: 36063381 Free PMC article.
-
Emerging mechanistic understanding of cilia function in cellular signalling.Nat Rev Mol Cell Biol. 2024 Jul;25(7):555-573. doi: 10.1038/s41580-023-00698-5. Epub 2024 Feb 16. Nat Rev Mol Cell Biol. 2024. PMID: 38366037 Free PMC article. Review.
-
Cilia at the crossroad: convergence of regulatory mechanisms to govern cilia dynamics during cell signaling and the cell cycle.Cell Biosci. 2025 Jun 7;15(1):81. doi: 10.1186/s13578-025-01403-z. Cell Biosci. 2025. PMID: 40483459 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

