The Role of Histone Protein Acetylation in Regulating Endothelial Function
- PMID: 33996829
- PMCID: PMC8113824
- DOI: 10.3389/fcell.2021.672447
The Role of Histone Protein Acetylation in Regulating Endothelial Function
Abstract
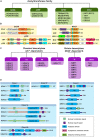
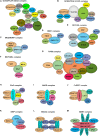
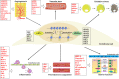
Endothelial cell (EC), consisting of the innermost cellular layer of all types of vessels, is not only a barrier composer but also performing multiple functions in physiological processes. It actively controls the vascular tone and the extravasation of water, solutes, and macromolecules; modulates circulating immune cells as well as platelet and leukocyte recruitment/adhesion and activation. In addition, EC also tightly keeps coagulation/fibrinolysis balance and plays a major role in angiogenesis. Therefore, endothelial dysfunction contributes to the pathogenesis of many diseases. Growing pieces of evidence suggest that histone protein acetylation, an epigenetic mark, is altered in ECs under different conditions, and the acetylation status change at different lysine sites on histone protein plays a key role in endothelial dysfunction and involved in hyperglycemia, hypertension, inflammatory disease, cancer and so on. In this review, we highlight the importance of histone acetylation in regulating endothelial functions and discuss the roles of histone acetylation across the transcriptional unit of protein-coding genes in ECs under different disease-related pathophysiological processes. Since histone acetylation changes are conserved and reversible, the knowledge of histone acetylation in endothelial function regulation could provide insights to develop epigenetic interventions in preventing or treating endothelial dysfunction-related diseases.
Keywords: acetyltransferase; deacetylase; endothelial dysfunction; epigenetic regulation; histone acetylation.
Copyright © 2021 Fang, Wang, Sun, Hu and Miao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Advani A., Huang Q., Thai K., Advani S. L., White K. E., Kelly D. J., et al. (2011). Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am. J. Pathol. 178 2205–2214. 10.1016/j.ajpath.2011.01.044 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

