Short-Term Outcomes of Refractory Diabetic Macular Edema Switch From Ranibizumab to Dexamethasone Implant and the Influential Factors: A Retrospective Real World Experience
- PMID: 33996856
- PMCID: PMC8121145
- DOI: 10.3389/fmed.2021.649979
Short-Term Outcomes of Refractory Diabetic Macular Edema Switch From Ranibizumab to Dexamethasone Implant and the Influential Factors: A Retrospective Real World Experience
Abstract
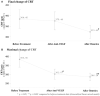
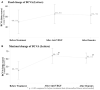
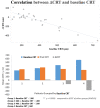
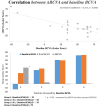
Introduction: To evaluate the effectiveness and safety of intravitreal dexamethasone (DEX) implants in refractory diabetic macular edema (DME) treated by intravitreal ranibizumab. Materials and Methods: We retrospectively analyzed DME patients who received DEX implant treatment after being refractory to at least 3 monthly intravitreal ranibizumab injections. The main outcomes were best-corrected visual acuity (BCVA), central retinal thickness (CRT), and intraocular pressure (IOP). Results: Twenty-nine eyes of 26 patients who had previously received an average of 8.1 ± 4.4 ranibizumab injections were included. Patients received between one and three DEX implants during 12.4 ± 7.4 months of follow-up. The mean final CRT significantly decreased from 384.4 ± 114.4 μm at baseline to 323.9 ± 77.7 μm (p = 0.0249). The mean final BCVA was 51.4 ± 21.3 letters, which was not significant compared to baseline (44.9 ± 30.2 letters, p = 0.1149). Mean IOP did not increase significantly. All patients tolerated the treatment well without serious adverse events. Higher baseline CRT and worse BCVA correlated with better therapeutic responses. Conclusion: Switching to DEX implant is feasible and safe for treating patients of DME refractory to intravitreal ranibizumab in real world. Further larger-scale or multicenter studies would be conducted to explore different DEX treatment strategies for DME, such as first-line or early switch therapy, for better BCVA improvement.
Keywords: diabetic macular edema; intravitreal dexamethasone implant; intravitreal ranibizumab; ozurdex; refractory diabetic macular edema.
Copyright © 2021 Hsia, Lin, Chen, Chang, Bair, Lai, Lin, Chen, Tien, Wu and Tsai.
Conflict of interest statement
H-SC is employed by company NephroCare Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema.Graefes Arch Clin Exp Ophthalmol. 2017 Mar;255(3):463-473. doi: 10.1007/s00417-016-3472-1. Epub 2016 Sep 8. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 27632215 Clinical Trial.
-
Comparison of early dexamethasone retreatment versus standard dexamethasone regimen combined with PRN ranibizumab in diabetic macular edema.Int Ophthalmol. 2017 Feb;37(1):185-196. doi: 10.1007/s10792-016-0251-2. Epub 2016 May 12. Int Ophthalmol. 2017. PMID: 27173834
-
Two-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France.Graefes Arch Clin Exp Ophthalmol. 2016 Dec;254(12):2307-2318. doi: 10.1007/s00417-016-3394-y. Epub 2016 Jun 11. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27286894 Free PMC article. Clinical Trial.
-
Practical Lessons from Protocol I for the Management of Diabetic Macular Edema.Dev Ophthalmol. 2017;60:91-108. doi: 10.1159/000459692. Epub 2017 Apr 20. Dev Ophthalmol. 2017. PMID: 28427069 Review.
-
Comparison between Ozurdex and intravitreal anti-vascular endothelial growth factor treatment for retinal vein occlusion-related macular edema: A systematic review and meta-analysis of randomized controlled trials.Indian J Ophthalmol. 2019 Nov;67(11):1800-1809. doi: 10.4103/ijo.IJO_382_19. Indian J Ophthalmol. 2019. PMID: 31638037 Free PMC article.
Cited by
-
Early-switch versus late-switch in patients with diabetic macular edema: a cost-effectiveness study.Graefes Arch Clin Exp Ophthalmol. 2023 Apr;261(4):941-949. doi: 10.1007/s00417-022-05892-3. Epub 2022 Nov 12. Graefes Arch Clin Exp Ophthalmol. 2023. PMID: 36370170 Free PMC article.
-
Optical Coherence Tomography Biomarkers in Predicting Treatment Outcomes of Diabetic Macular Edema After Dexamethasone Implants.Front Med (Lausanne). 2022 Jun 9;9:852022. doi: 10.3389/fmed.2022.852022. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35755055 Free PMC article.
-
Dexamethasone implant in naive versus refractory patients with diabetic macular edema: a Meta-analysis.Int J Ophthalmol. 2024 Oct 18;17(10):1898-1904. doi: 10.18240/ijo.2024.10.17. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430021 Free PMC article.
-
Comparison of Optical Coherence Tomography Biomarkers between Bevacizumab Good Responders and Nonresponders Who were Switched to Dexamethasone Implant in Diabetic Macular Edema.Korean J Ophthalmol. 2023 Apr;37(2):137-146. doi: 10.3341/kjo.2022.0109. Epub 2023 Mar 23. Korean J Ophthalmol. 2023. PMID: 36950923 Free PMC article.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 8th edn. Brussels: International Diabetes Federation; (2017).
-
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. . Diabetic retinopathy clinical research network. aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. (2016) 123:1351–9. 10.1016/j.ophtha.2016.02.022 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

