Short-Term Outcomes of Refractory Diabetic Macular Edema Switch From Ranibizumab to Dexamethasone Implant and the Influential Factors: A Retrospective Real World Experience
- PMID: 33996856
- PMCID: PMC8121145
- DOI: 10.3389/fmed.2021.649979
Short-Term Outcomes of Refractory Diabetic Macular Edema Switch From Ranibizumab to Dexamethasone Implant and the Influential Factors: A Retrospective Real World Experience
Abstract
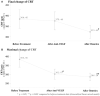
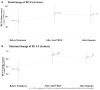
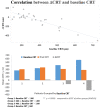
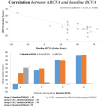
Introduction: To evaluate the effectiveness and safety of intravitreal dexamethasone (DEX) implants in refractory diabetic macular edema (DME) treated by intravitreal ranibizumab. Materials and Methods: We retrospectively analyzed DME patients who received DEX implant treatment after being refractory to at least 3 monthly intravitreal ranibizumab injections. The main outcomes were best-corrected visual acuity (BCVA), central retinal thickness (CRT), and intraocular pressure (IOP). Results: Twenty-nine eyes of 26 patients who had previously received an average of 8.1 ± 4.4 ranibizumab injections were included. Patients received between one and three DEX implants during 12.4 ± 7.4 months of follow-up. The mean final CRT significantly decreased from 384.4 ± 114.4 μm at baseline to 323.9 ± 77.7 μm (p = 0.0249). The mean final BCVA was 51.4 ± 21.3 letters, which was not significant compared to baseline (44.9 ± 30.2 letters, p = 0.1149). Mean IOP did not increase significantly. All patients tolerated the treatment well without serious adverse events. Higher baseline CRT and worse BCVA correlated with better therapeutic responses. Conclusion: Switching to DEX implant is feasible and safe for treating patients of DME refractory to intravitreal ranibizumab in real world. Further larger-scale or multicenter studies would be conducted to explore different DEX treatment strategies for DME, such as first-line or early switch therapy, for better BCVA improvement.
Keywords: diabetic macular edema; intravitreal dexamethasone implant; intravitreal ranibizumab; ozurdex; refractory diabetic macular edema.
Copyright © 2021 Hsia, Lin, Chen, Chang, Bair, Lai, Lin, Chen, Tien, Wu and Tsai.
Conflict of interest statement
H-SC is employed by company NephroCare Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 8th edn. Brussels: International Diabetes Federation; (2017).
-
- Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. . Diabetic retinopathy clinical research network. aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. (2016) 123:1351–9. 10.1016/j.ophtha.2016.02.022 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

