Hiding in Plain Sight: Formation and Function of Stress Granules During Microbial Infection of Mammalian Cells
- PMID: 33996904
- PMCID: PMC8116797
- DOI: 10.3389/fmolb.2021.647884
Hiding in Plain Sight: Formation and Function of Stress Granules During Microbial Infection of Mammalian Cells
Abstract
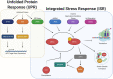
Stress granule (SG) formation is a host cell response to stress-induced translational repression. SGs assemble with RNA-binding proteins and translationally silent mRNA. SGs have been demonstrated to be both inhibitory to viruses, as well as being subverted for viral roles. In contrast, the function of SGs during non-viral microbial infections remains largely unexplored. A handful of microbial infections have been shown to result in host SG assembly. Nevertheless, a large body of evidence suggests SG formation in hosts is a widespread response to microbial infection. Diverse stresses caused by microbes and their products can activate the integrated stress response in order to inhibit translation initiation through phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α). This translational response in other contexts results in SG assembly, suggesting that SG assembly can be a general phenomenon during microbial infection. This review explores evidence for host SG formation in response to bacterial, fungal, and protozoan infection and potential functions of SGs in the host and for adaptations of the pathogen.
Keywords: GCN2; HRI; PERK; PKR; eIF2 alpha; integrated stress response (ISR); stress granules (SG); unfolded protein response (UPR).
Copyright © 2021 Tweedie and Nissan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mouse Norovirus Infection Arrests Host Cell Translation Uncoupled from the Stress Granule-PKR-eIF2α Axis.mBio. 2019 Jun 18;10(3):e00960-19. doi: 10.1128/mBio.00960-19. mBio. 2019. PMID: 31213553 Free PMC article.
-
Defining the Role of Stress Granules in Innate Immune Suppression by the Herpes Simplex Virus 1 Endoribonuclease VHS.J Virol. 2018 Jul 17;92(15):e00829-18. doi: 10.1128/JVI.00829-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793959 Free PMC article.
-
Ebola Virus Does Not Induce Stress Granule Formation during Infection and Sequesters Stress Granule Proteins within Viral Inclusions.J Virol. 2016 Jul 27;90(16):7268-7284. doi: 10.1128/JVI.00459-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252530 Free PMC article.
-
The role of host eIF2α in viral infection.Virol J. 2020 Jul 23;17(1):112. doi: 10.1186/s12985-020-01362-6. Virol J. 2020. PMID: 32703221 Free PMC article. Review.
-
Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation.Cell Stress Chaperones. 2002 Apr;7(2):213-21. doi: 10.1379/1466-1268(2002)007<0213:vstroe>2.0.co;2. Cell Stress Chaperones. 2002. PMID: 12380690 Free PMC article. Review.
Cited by
-
Establishment of a stress granule reporter system for evaluating in vitro colon toxicity.Anim Cells Syst (Seoul). 2024 Jun 17;28(1):315-325. doi: 10.1080/19768354.2024.2364673. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 38895161 Free PMC article.
-
Stress granules: potential therapeutic targets for infectious and inflammatory diseases.Front Immunol. 2023 May 2;14:1145346. doi: 10.3389/fimmu.2023.1145346. eCollection 2023. Front Immunol. 2023. PMID: 37205103 Free PMC article. Review.
-
Visualizing stress granule dynamics with an RNA guanine quadruplex targeted ruthenium(ii) peptide conjugate.RSC Chem Biol. 2025 Jun 19;6(9):1403-13. doi: 10.1039/d5cb00008d. Online ahead of print. RSC Chem Biol. 2025. PMID: 40575133 Free PMC article.
-
Cytosolic Phospholipase A2 Determines Intercellular Heterogeneity of Stress Granules and Chemotherapy Response.Cancer Discov. 2025 Jul 3;15(7):1437-1457. doi: 10.1158/2159-8290.CD-24-1144. Cancer Discov. 2025. PMID: 40099649
-
Melatonin: Regulation of Viral Phase Separation and Epitranscriptomics in Post-Acute Sequelae of COVID-19.Int J Mol Sci. 2022 Jul 23;23(15):8122. doi: 10.3390/ijms23158122. Int J Mol Sci. 2022. PMID: 35897696 Free PMC article. Review.
References
-
- Anderson P., Kedersha N. (2002). Stressful initiations. J. Cell Sci. 115:3227. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

