The Protein Quality Control Network in Caulobacter crescentus
- PMID: 33996917
- PMCID: PMC8119881
- DOI: 10.3389/fmolb.2021.682967
The Protein Quality Control Network in Caulobacter crescentus
Abstract
The asymmetric life cycle of Caulobacter crescentus has provided a model in which to study how protein quality control (PQC) networks interface with cell cycle and developmental processes, and how the functions of these systems change during exposure to stress. As in most bacteria, the PQC network of Caulobacter contains highly conserved ATP-dependent chaperones and proteases as well as more specialized holdases. During growth in optimal conditions, these systems support a regulated circuit of protein synthesis and degradation that drives cell differentiation and cell cycle progression. When stress conditions threaten the proteome, most components of the Caulobacter proteostasis network are upregulated and switch to survival functions that prevent, revert, and remove protein damage, while simultaneously pausing the cell cycle in order to regain protein homeostasis. The specialized physiology of Caulobacter influences how it copes with proteotoxic stress, such as in the global management of damaged proteins during recovery as well as in cell type-specific stress responses. Our mini-review highlights the discoveries that have been made in how Caulobacter utilizes its PQC network for regulating its life cycle under optimal and proteotoxic stress conditions, and discusses open research questions in this model.
Keywords: bacterial development; cell cycle; chaperone; holdase; protease; protein quality control.
Copyright © 2021 Schroeder and Jonas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
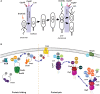
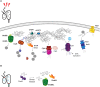
Similar articles
-
Regulated Proteolysis in Bacteria: Caulobacter.Annu Rev Genet. 2016 Nov 23;50:423-445. doi: 10.1146/annurev-genet-120215-035235. Epub 2016 Oct 13. Annu Rev Genet. 2016. PMID: 27893963 Free PMC article. Review.
-
The Chaperone and Redox Properties of CnoX Chaperedoxins Are Tailored to the Proteostatic Needs of Bacterial Species.mBio. 2018 Nov 27;9(6):e01541-18. doi: 10.1128/mBio.01541-18. mBio. 2018. PMID: 30482828 Free PMC article.
-
Regulation of the replication initiator DnaA in Caulobacter crescentus.Biochim Biophys Acta Gene Regul Mech. 2019 Jul;1862(7):697-705. doi: 10.1016/j.bbagrm.2018.01.004. Epub 2018 Jan 31. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 29382570 Review.
-
GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus.J Bacteriol. 2006 Dec;188(23):8044-53. doi: 10.1128/JB.00824-06. Epub 2006 Sep 15. J Bacteriol. 2006. PMID: 16980445 Free PMC article.
-
An essential regulatory function of the DnaK chaperone dictates the decision between proliferation and maintenance in Caulobacter crescentus.PLoS Genet. 2017 Dec 27;13(12):e1007148. doi: 10.1371/journal.pgen.1007148. eCollection 2017 Dec. PLoS Genet. 2017. PMID: 29281627 Free PMC article.
Cited by
-
The Lon protease temporally restricts polar cell differentiation events during the Caulobacter cell cycle.Elife. 2021 Oct 25;10:e73875. doi: 10.7554/eLife.73875. Elife. 2021. PMID: 34693909 Free PMC article.
-
Discovering genetic modulators of the protein homeostasis system through multilevel analysis.PNAS Nexus. 2025 Jan 13;4(1):pgae574. doi: 10.1093/pnasnexus/pgae574. eCollection 2025 Jan. PNAS Nexus. 2025. PMID: 39807344 Free PMC article.
-
Stress testing reveals selective vulnerabilities in protein homeostasis.bioRxiv [Preprint]. 2025 Jun 16:2025.06.11.659168. doi: 10.1101/2025.06.11.659168. bioRxiv. 2025. PMID: 40666924 Free PMC article. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

