Prenatal Management Strategy for Immune-Associated Congenital Heart Block in Fetuses
- PMID: 33996939
- PMCID: PMC8113399
- DOI: 10.3389/fcvm.2021.644122
Prenatal Management Strategy for Immune-Associated Congenital Heart Block in Fetuses
Abstract
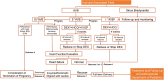
Fetal congenital heart block (CHB) is the most commonly observed type of fetal bradycardia, and is potentially life-threatening. More than 50% of cases of bradycardia are associated with maternal autoimmunity, and these are collectively termed immune-associated bradycardia. Several methods have been used to achieve reliable prenatal diagnoses of CHB. Emerging data and opinions on pathogenesis, prenatal diagnosis, fetal intervention, and the prognosis of fetal immune-associated CHB provide clues for generating a practical protocol for clinical management. The prognosis of fetal immune-associated bradycardia is based on the severity of heart blocks. Morbidity and mortality can occur in severe cases, thus hieratical management is essential in such cases. In this review, we mainly focus on optimal strategies pertaining to autoimmune antibodies related to CHB, although the approaches for managing autoimmune-mediated CHB are still controversial, particularly with regard to whether fetuses benefit from transplacental medication administration. To date there is still no accessible clinical strategy for autoimmune-mediated CHB. This review first discusses integrated prenatal management strategies for the condition. It then provides some advice for clinicians involved in management of fetal cardiovascular disorder.
Keywords: fetal immune-associated heart block; outcome; prenatal diagnosis; prenatal management; transplacental drug administration.
Copyright © 2021 Liao, Tang, Qiao, Zhou, Hua, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Autoimmune-associated Congenital Heart Block: A New Insight in Fetal Life.Chin Med J (Engl). 2017 Dec 5;130(23):2863-2871. doi: 10.4103/0366-6999.219160. Chin Med J (Engl). 2017. PMID: 29176145 Free PMC article. Review.
-
[Congenital heart block associated with maternal anti SSA/SSB antibodies :a report of four cases].Pathol Biol (Paris). 2004 Apr;52(3):138-47. doi: 10.1016/j.patbio.2003.06.002. Pathol Biol (Paris). 2004. PMID: 15063933 Review. French.
-
Autoimmune Congenital Heart Block: A Review of Biomarkers and Management of Pregnancy.Front Pediatr. 2020 Dec 22;8:607515. doi: 10.3389/fped.2020.607515. eCollection 2020. Front Pediatr. 2020. PMID: 33415090 Free PMC article. Review.
-
Fetal arrhythmia: prenatal diagnosis and perinatal management.J Obstet Gynaecol Res. 2009 Aug;35(4):623-9. doi: 10.1111/j.1447-0756.2009.01080.x. J Obstet Gynaecol Res. 2009. PMID: 19751319 Review.
-
Congenital heart block in neonatal lupus: the pediatric cardiologist's perspective.Indian J Pediatr. 2002 Jun;69(6):517-22. doi: 10.1007/BF02722656. Indian J Pediatr. 2002. PMID: 12139139 Review.
Cited by
-
Dual challenge inside the womb: a case report of concomitant fetal atrio-ventricular block associated with maternal anti-SSA antibodies and fetal tachyarrhythmia diagnosed as Wolff-Parkinson-White syndrome after birth.Front Immunol. 2024 Jul 24;15:1397103. doi: 10.3389/fimmu.2024.1397103. eCollection 2024. Front Immunol. 2024. PMID: 39114649 Free PMC article.
-
Monitoring of Women with Anti-Ro/SSA and Anti-La/SSB Antibodies in Germany-Status Quo and Intensified Monitoring Concepts.J Clin Med. 2024 Feb 17;13(4):1142. doi: 10.3390/jcm13041142. J Clin Med. 2024. PMID: 38398455 Free PMC article.
-
Autoimmune Disease Classification Based on PubMed Text Mining.J Clin Med. 2022 Jul 26;11(15):4345. doi: 10.3390/jcm11154345. J Clin Med. 2022. PMID: 35893435 Free PMC article.
-
Case Report: A novel KNCH2 variant-induced fetal heart block and the advantages of fetal genomic sequencing in prenatal long-term dexamethasone exposure.Front Genet. 2022 Nov 29;13:1010078. doi: 10.3389/fgene.2022.1010078. eCollection 2022. Front Genet. 2022. PMID: 36523767 Free PMC article.
-
Case Report: Prenatal Diagnosis and Treatment of Fetal Autoimmune-Associated First-Degree Atrioventricular Block: First Report From China.Front Cardiovasc Med. 2021 Jun 21;8:683486. doi: 10.3389/fcvm.2021.683486. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34235189 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

