Development of Perphenazine-Loaded Solid Lipid Nanoparticles: Statistical Optimization and Cytotoxicity Studies
- PMID: 33997026
- PMCID: PMC8099510
- DOI: 10.1155/2021/6619195
Development of Perphenazine-Loaded Solid Lipid Nanoparticles: Statistical Optimization and Cytotoxicity Studies
Abstract
Objective: Perphenazine (PPZ), as a typical antipsychotic medical substance, has the same effectiveness compared to atypical antipsychotic medications for the treatment of schizophrenia. Despite the lipophilic essence, PPZ encounters limited bioavailability caused by the first-pass metabolism following oral administration. In the present study, PPZ-containing solid lipid nanoparticles (PPZ-SLNs) were prepared and optimized based on different factors, including lipid and surfactant amount, to develop appropriate and safe novel oral dosage forms of PPZ.
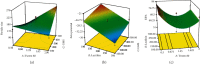
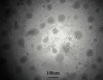
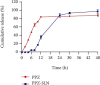
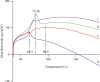
Methods: The solvent emulsification-evaporation method was utilized to form SLNs by using soybean lecithin, glycerol monostearate (GMS), and Tween 80. Statistical optimization was done by the Box-Behnken design method to achieve formulation with optimized particle size, entrapment efficiency, and zeta potential. Also, transmission electron microscopy, in vitro release behavior, differential scanning calorimetry (DSC), and powder X-ray diffractometry (P-XRD) studies and cytotoxicity studies were assessed.
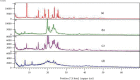
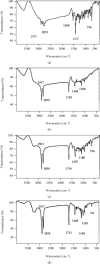
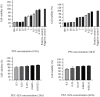
Results: Optimization exhibited the significant effect of various excipients on SLN characteristics. Our finding indicated that the mean particle size, zeta potential, and entrapment efficiency of optimized PPZ-SLN were, respectively, 104 ± 3.92 nm, -28 ± 2.28 mV, and 83% ± 1.29. Drug release of PPZ-SLN was observed to be greater than 90% for 48 h that emphasized a sustained-release pattern. The DSC and P-XRD studies revealed the amorphous state of PPZ-SLN. FTIR spectra showed no incompatibility between the drug and the lipid. Performing cytotoxicity studies indicated no significant cytotoxicity on HT-29 cell culture.
Conclusion: Our study suggests that PPZ-SLNs can make a promising vehicle for a suitable therapy of schizophrenia for the oral drug delivery system.
Copyright © 2021 Parisa Abbasi Farsani et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Preparation, characterization, and in vivo evaluation of perphenazine-loaded nanostructured lipid carriers for oral bioavailability improvement.Drug Dev Ind Pharm. 2021 Mar;47(3):509-520. doi: 10.1080/03639045.2021.1892745. Epub 2021 Mar 9. Drug Dev Ind Pharm. 2021. PMID: 33650445
-
Pharmacokinetics and brain distribution studies of perphenazine-loaded solid lipid nanoparticles.Drug Dev Ind Pharm. 2021 Jan;47(1):146-152. doi: 10.1080/03639045.2020.1862172. Epub 2020 Dec 18. Drug Dev Ind Pharm. 2021. PMID: 33307865
-
Part I: Development and optimization of solid-lipid nanoparticles using Box-Behnken statistical design for ocular delivery of gatifloxacin.J Biomed Mater Res A. 2013 Jun;101(6):1813-27. doi: 10.1002/jbm.a.34453. Epub 2012 Dec 18. J Biomed Mater Res A. 2013. PMID: 23255511
-
Development and characterization of Brigatinib loaded solid lipid nanoparticles: In-vitro cytotoxicity against human carcinoma A549 lung cell lines.Chem Phys Lipids. 2020 Nov;233:105003. doi: 10.1016/j.chemphyslip.2020.105003. Epub 2020 Oct 20. Chem Phys Lipids. 2020. PMID: 33096096
-
Sustained release of Ambrisentan solid lipid nanoparticles for the treatment of hypertension: Melt emulsification method.Ann Pharm Fr. 2025 Jun;83(4):649-663. doi: 10.1016/j.pharma.2025.01.003. Epub 2025 Jan 7. Ann Pharm Fr. 2025. PMID: 39788283
Cited by
-
Optimized Hesperidin-Loaded Lipid Nanoparticles with Tea Tree Oil for Enhanced Wound Healing: Formulation, Characterization, and Evaluation.Pharmaceuticals (Basel). 2025 Feb 20;18(3):290. doi: 10.3390/ph18030290. Pharmaceuticals (Basel). 2025. PMID: 40143069 Free PMC article.
-
Statistical optimization of tetrahydrocurcumin loaded solid lipid nanoparticles using Box Behnken design in the management of streptozotocin-induced diabetes mellitus.Saudi Pharm J. 2023 Sep;31(9):101727. doi: 10.1016/j.jsps.2023.101727. Epub 2023 Aug 2. Saudi Pharm J. 2023. PMID: 37638219 Free PMC article.
-
Analytical Quality by Design Driven Development and Validation of UV-Visible Spectrophotometric Method for Quantification of Xanthohumol in Bulk and Solid Lipid Nanoparticles.Turk J Pharm Sci. 2023 Jul 7;20(3):165-175. doi: 10.4274/tjps.galenos.2022.05335. Turk J Pharm Sci. 2023. PMID: 37417199 Free PMC article.
-
Gel-Dispersed Nanostructured Lipid Carriers Loading Thymol Designed for Dermal Pathologies.Int J Nanomedicine. 2024 Feb 7;19:1225-1248. doi: 10.2147/IJN.S433686. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38348173 Free PMC article.
References
-
- Lieberman J. A., Stroup T. S., Perkins D. O. Essentials of Schizophrenia. American Psychiatric Pub; 2012.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

